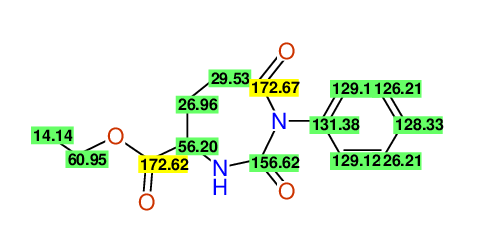
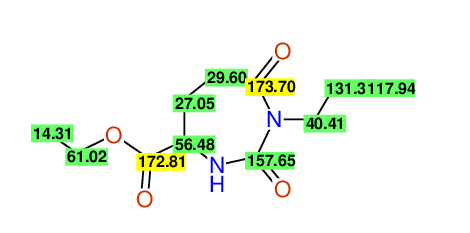
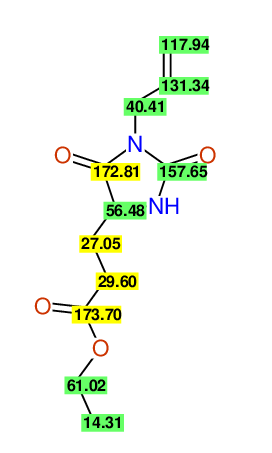
Comparison of 13C-NMR Data
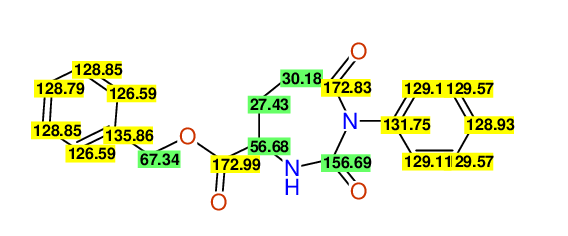
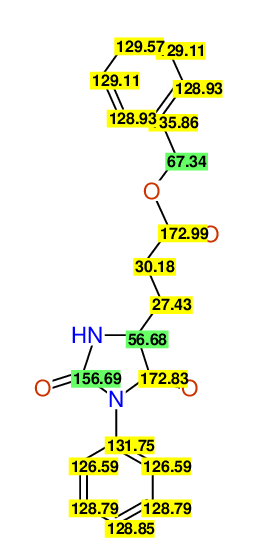
- Assigned lines as given in the publication are superimposed by a green rectangle
- Ambigiously assigned lines as given in the publication are superimposed by a yellow rectangle
- Ambigiously assigned lines are assigned to a specific carbon by us during data-input,
because of slightly different information
(e.g. Car versus Co,m)
slightly different assignments in the display may be obtained
- nevertheless the peaklist remains identical and the exchange-groups
are kept as given in the publication
|
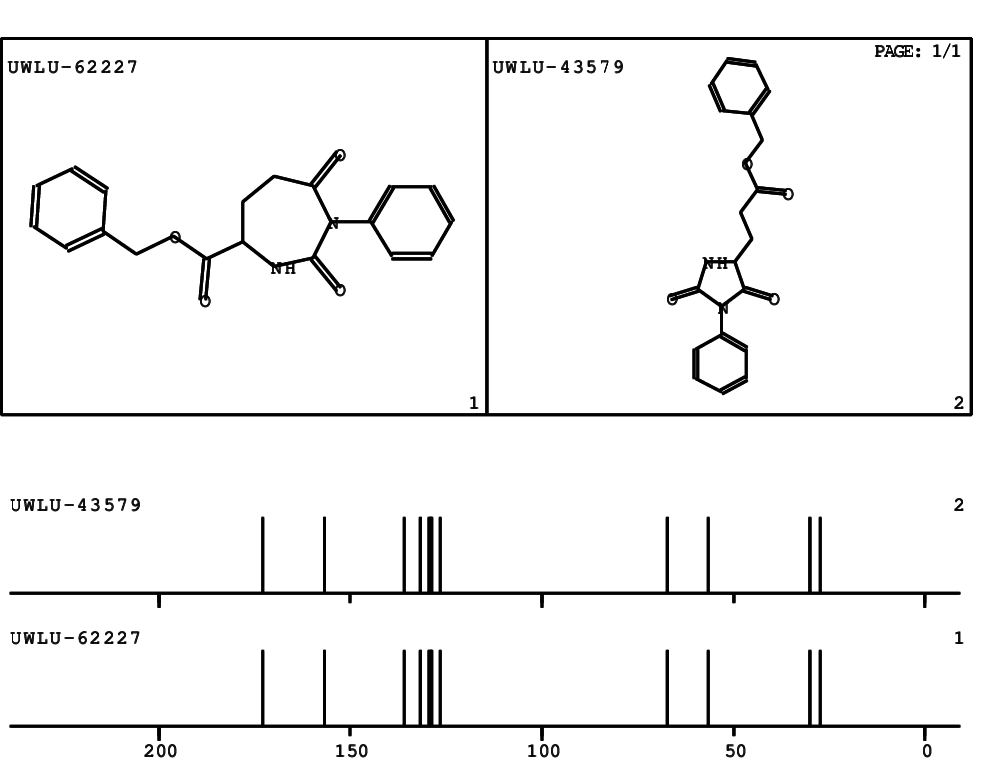
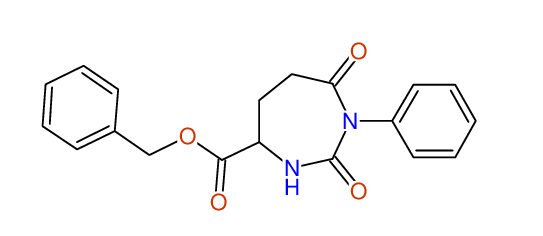
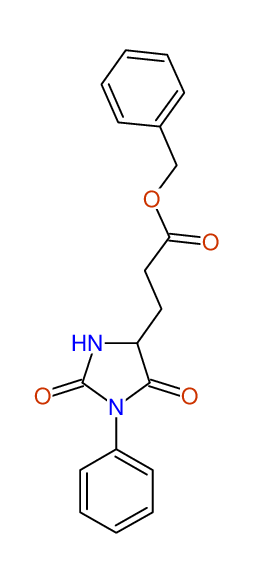
CSEARCH: UWLU-062227
Compound "Table 1 / Entry 1"
Org.Lett., 7, 1117 - 1119 (2005) |
CSEARCH: UWLU-043579
Compound 9A
Eur.J.Org.Chem.,2006,2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Identical melting point
- Structure revision
- Original literature not cited
|
|
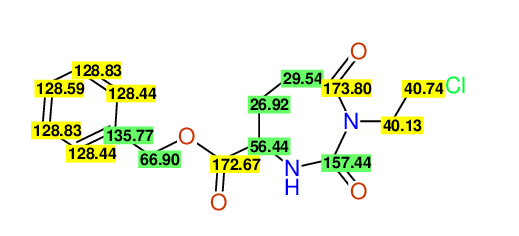
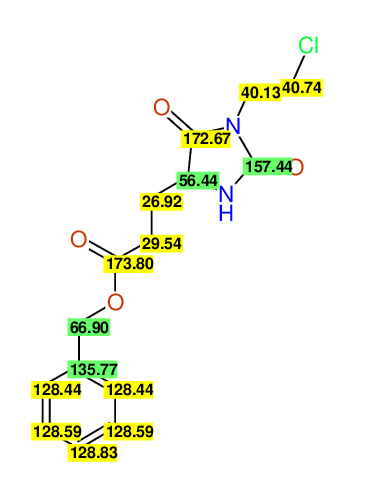
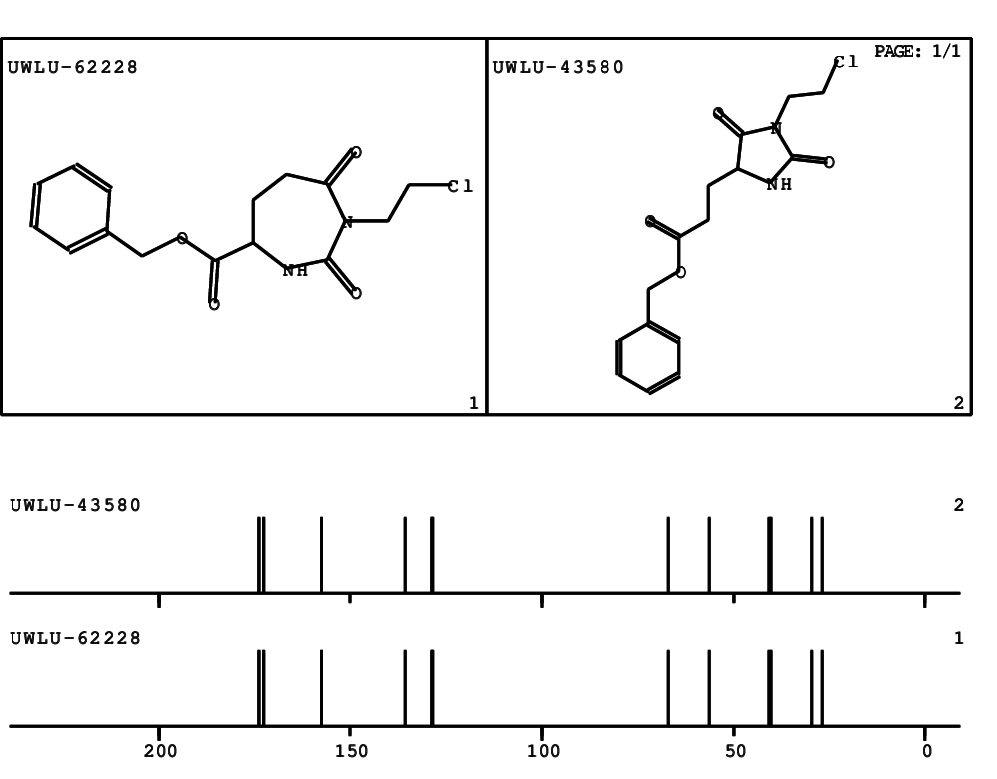
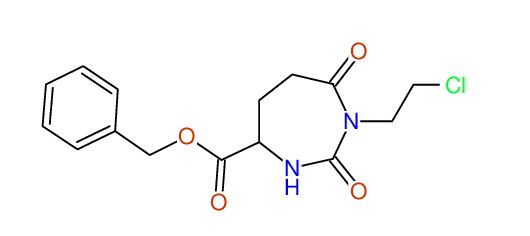
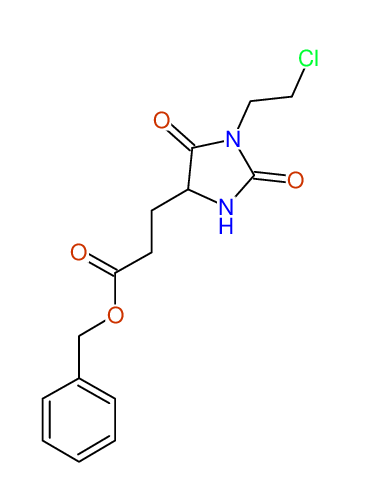
CSEARCH: UWLU-062228
Compound "Table 1 / Entry 2"
Org.Lett., 7, 1117 - 1119 (2005) |
CSEARCH: UWLU-043580
Compound 9B
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
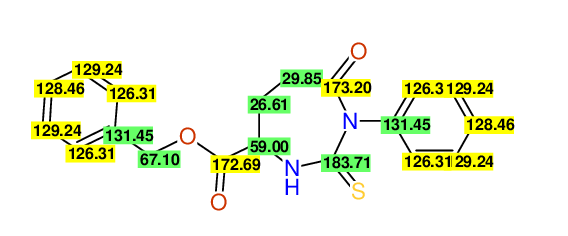
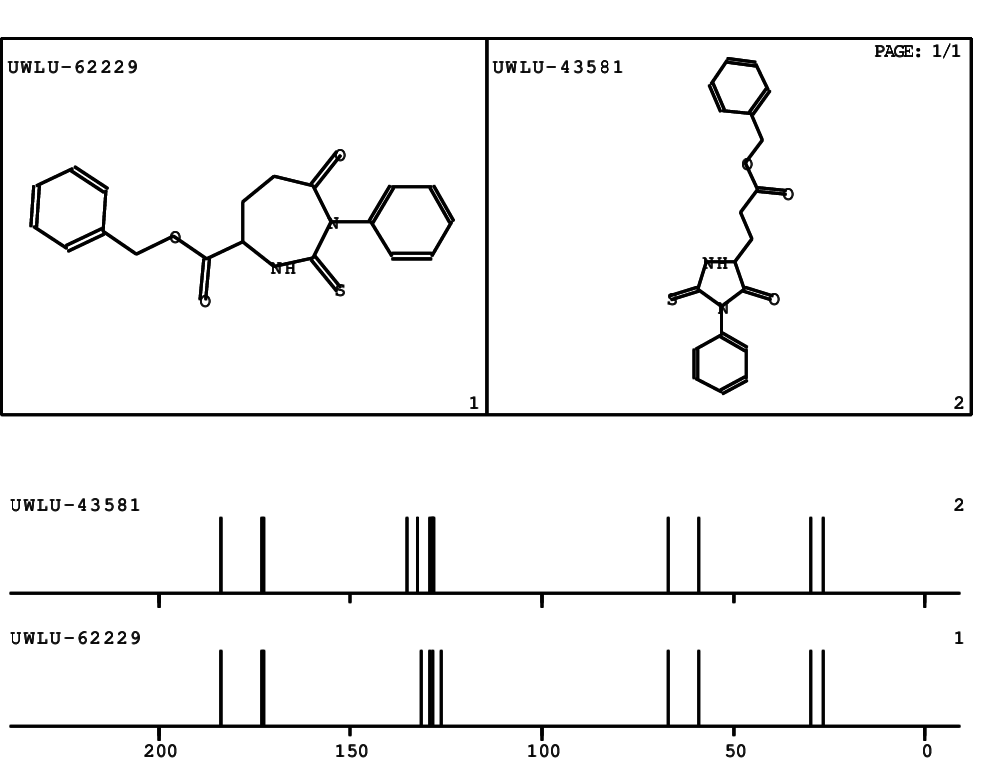
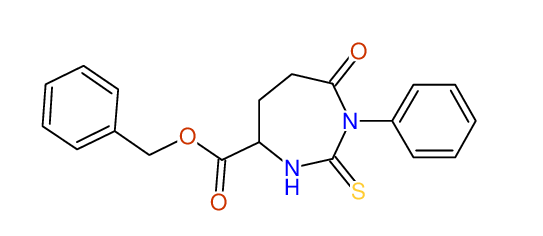
CSEARCH: UWLU-062229
Compound "Table 1 / Entry 3"
Org.Lett., 7, 1117 - 1119 (2005)
8 aromatic lines expected; 4 given
Melting point: 167oC |
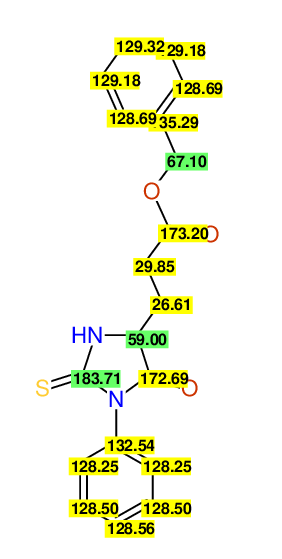
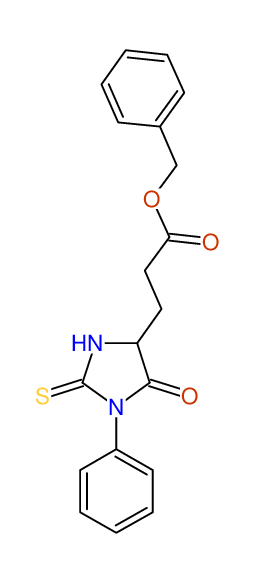
CSEARCH: UWLU-043581
Compound 9C
Eur.J.Org.Chem., 2006, 2649-2660
8 aromatic lines expected; 8 given
Melting point: 157oC
|
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Slightly different 13C-NMR data - see header
- Identical IR-data
- Melting point different - see header
- Structure revision
- Original literature not cited
|
|
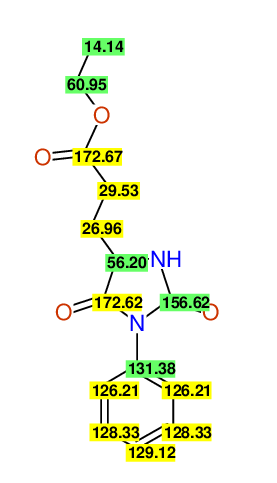
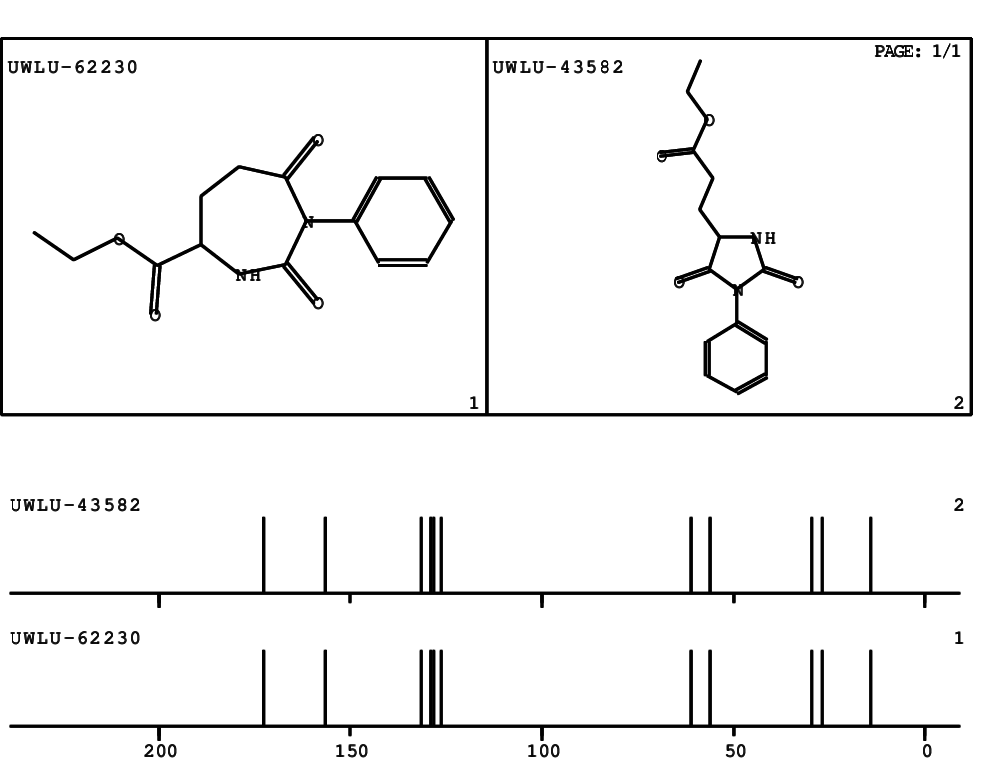
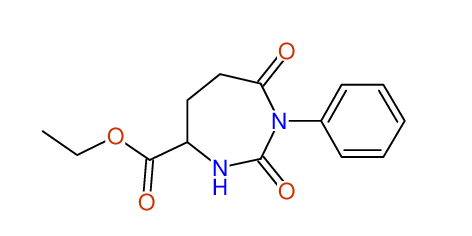
CSEARCH: UWLU-062230
Compound "Table 1 / Entry 4"
Org.Lett., 7, 1117 - 1119 (2005) |
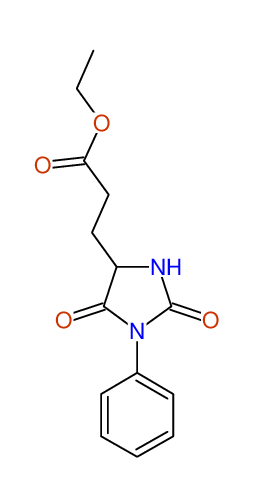
CSEARCH: UWLU-043582
Compound 9D
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Identical melting point
- Structure revision
- Original literature not cited
|
|
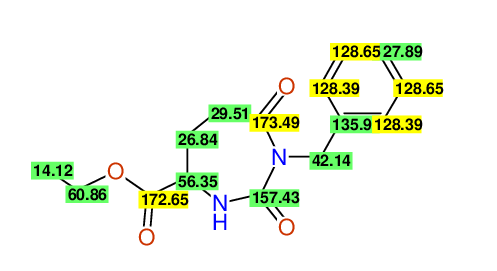
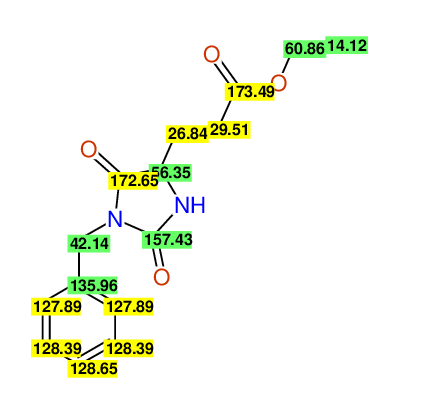
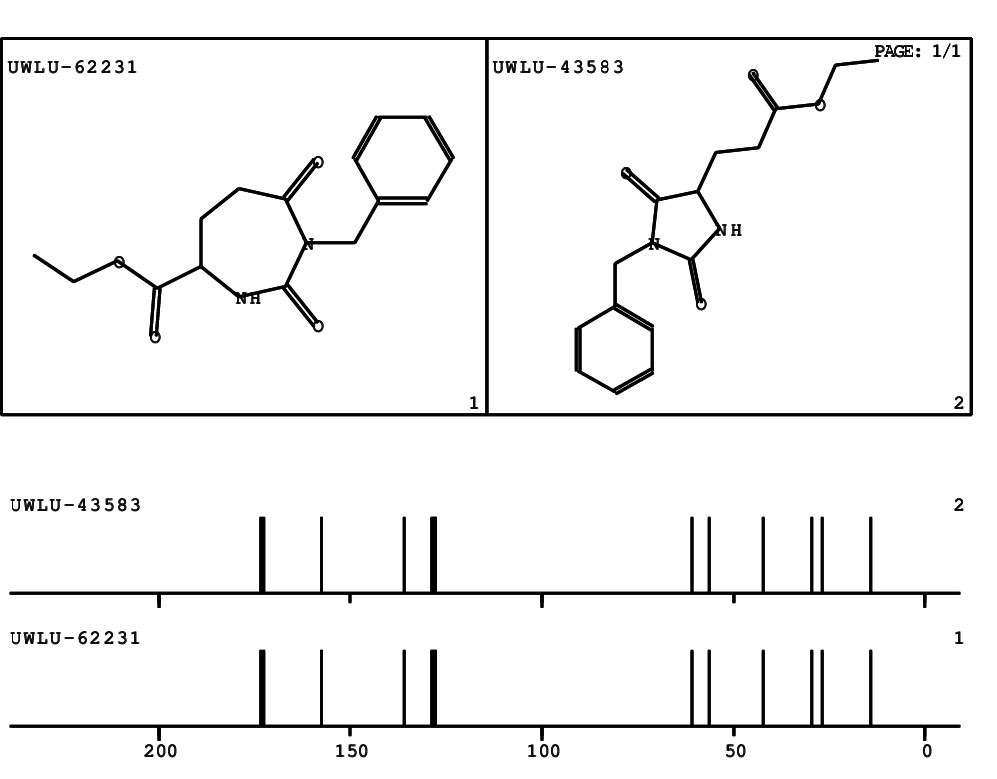
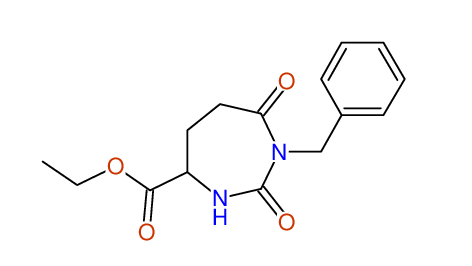
CSEARCH: UWLU-062231
Compound "Table 1 / Entry 5"
Org.Lett., 7, 1117 - 1119 (2005) |
CSEARCH: UWLU-043583
Compound 9E
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
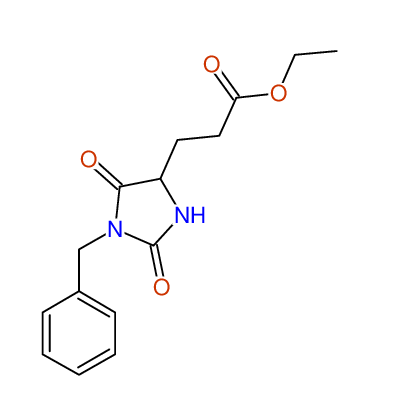 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Identical melting point
- Structure revision
- Original literature not cited
|
|
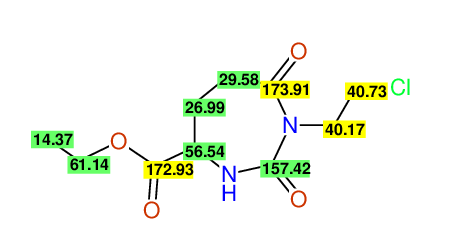
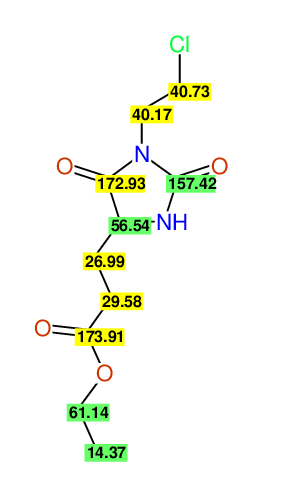
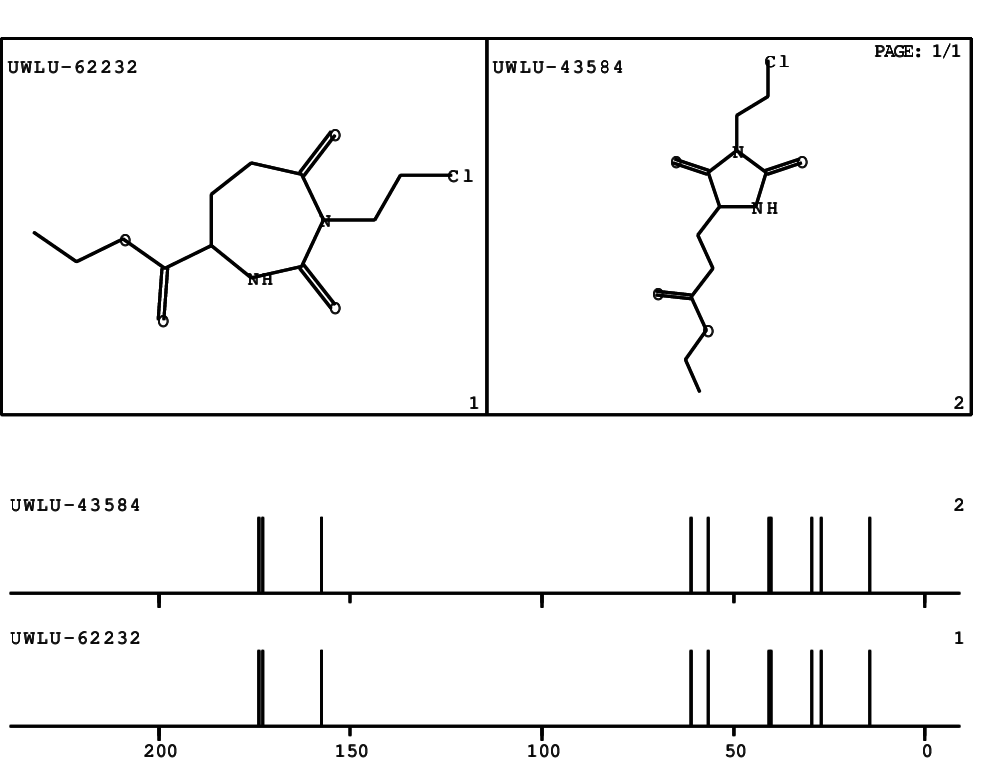
CSEARCH: UWLU-062232
Compound "Table 1 / Entry 6"
Org.Lett., 7, 1117 - 1119 (2005) |
CSEARCH: UWLU-043584
Compound 9F
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
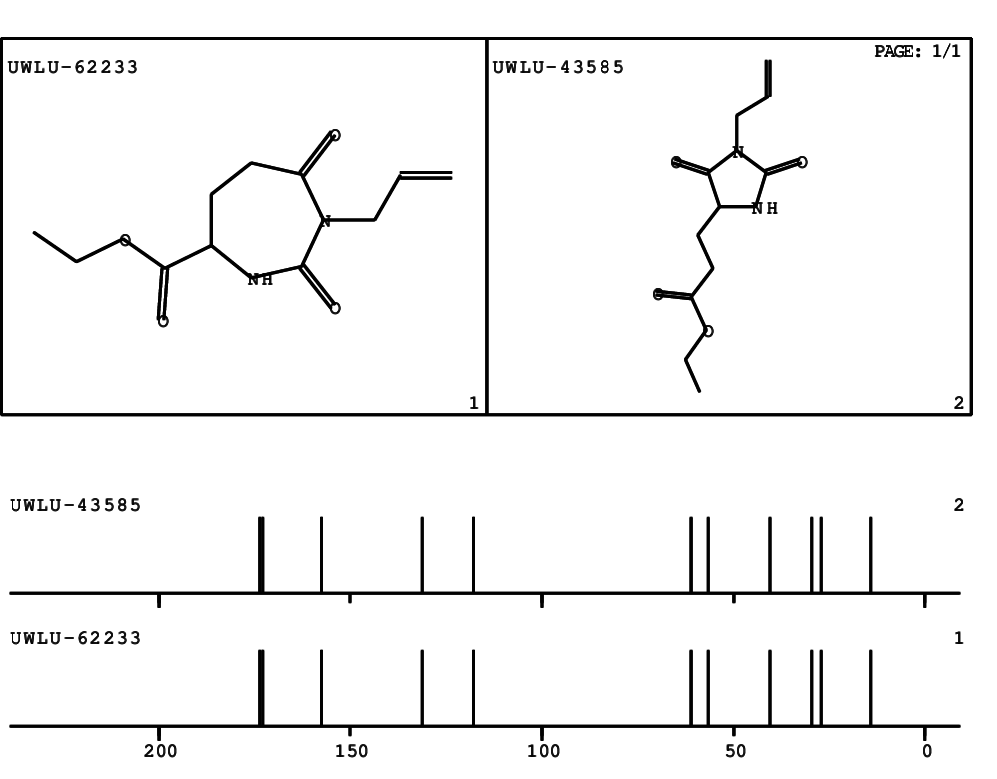
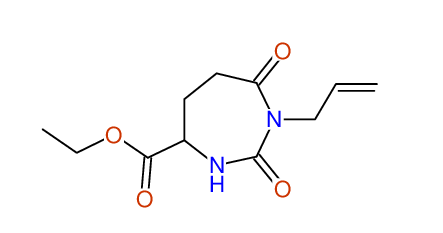
CSEARCH: UWLU-062233
Compound "Table 1 / Entry 7"
Org.Lett., 7, 1117 - 1119 (2005) |
CSEARCH: UWLU-043585
Compound 9G
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
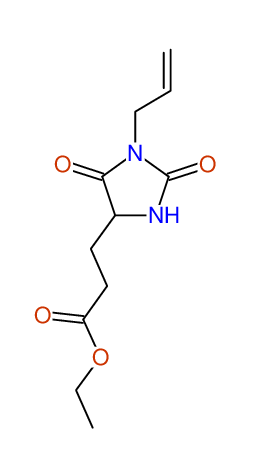 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
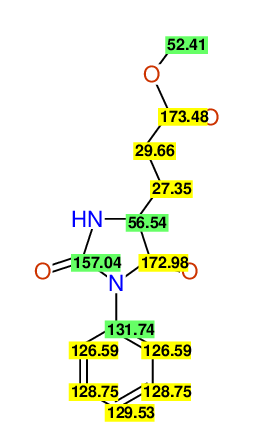
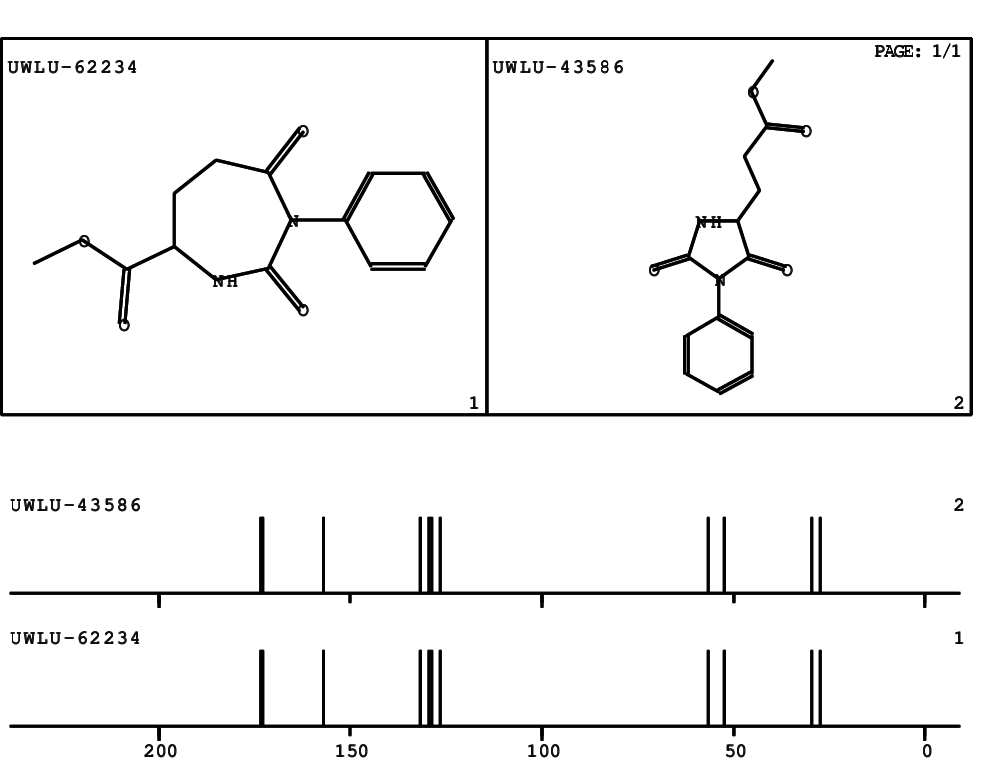
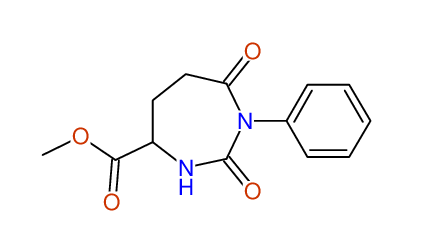
CSEARCH: UWLU-062234
Compound "Table 1 / Entry 8"
Org.Lett., 7, 1117 - 1119 (2005) |
CSEARCH: UWLU-043586
Compound 9H
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
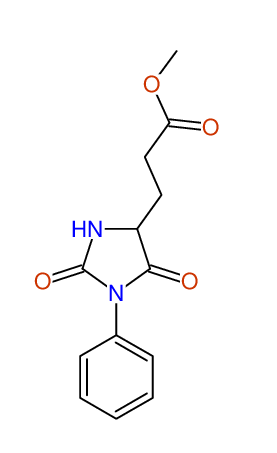 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Identical melting point
- Structure revision
- Original literature not cited
|
|
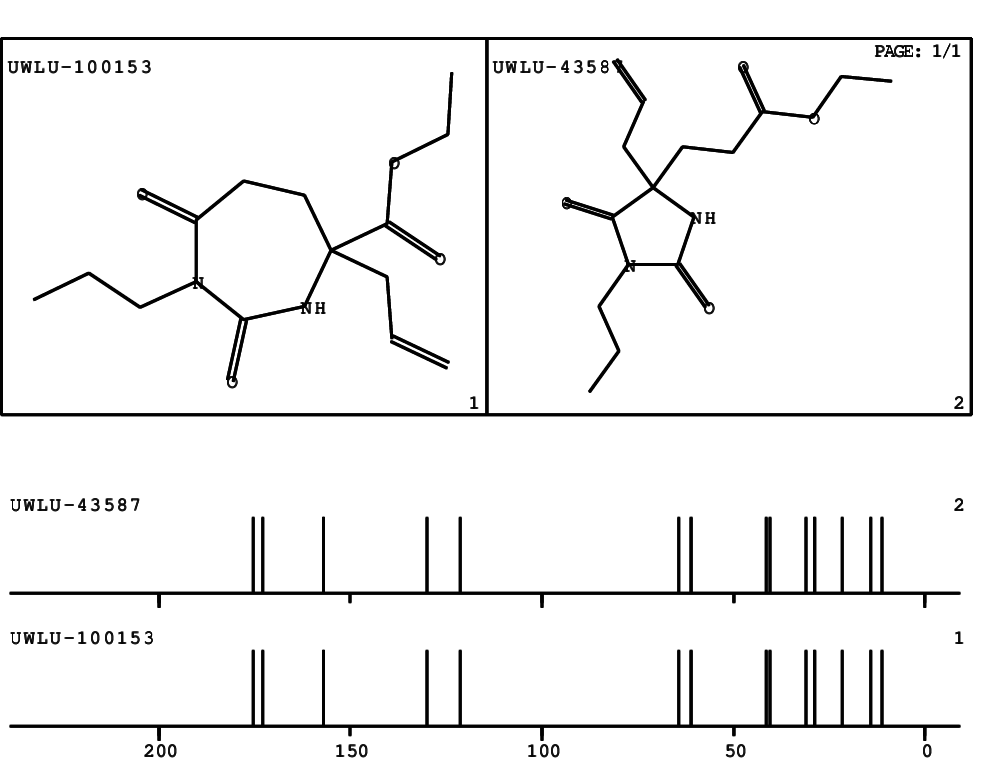
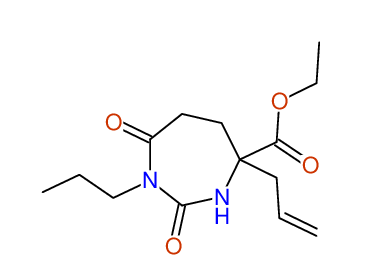
CSEARCH: UWLU-100153
ETHYL-4-ALLYL-2,7-DIOXO-1-PROPYL-1,3-DIAZEPANE-4-CARBOXYLATE
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043587
Compound 13A
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
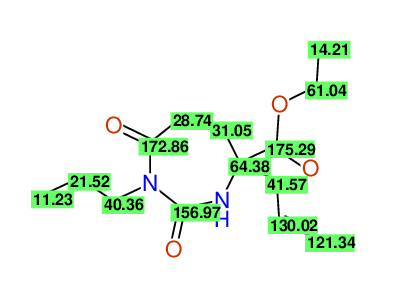 |
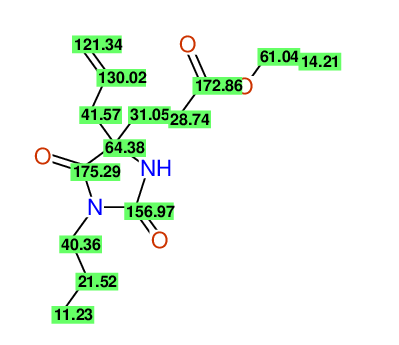 |
 |
 |
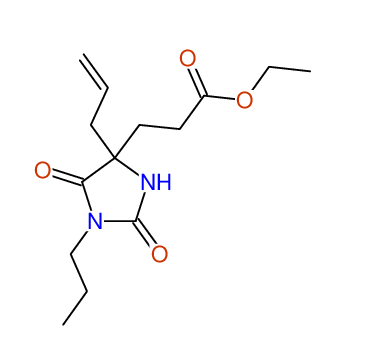 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
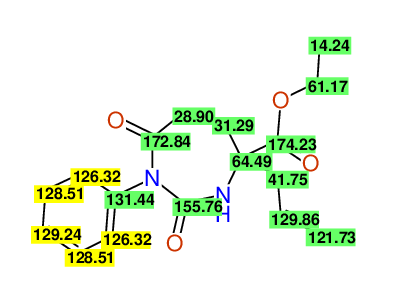
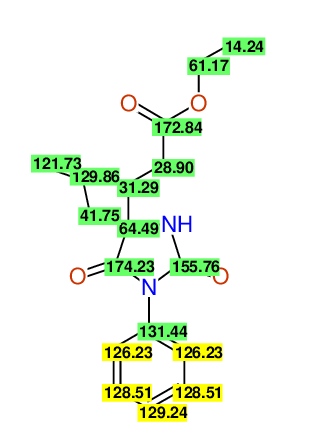
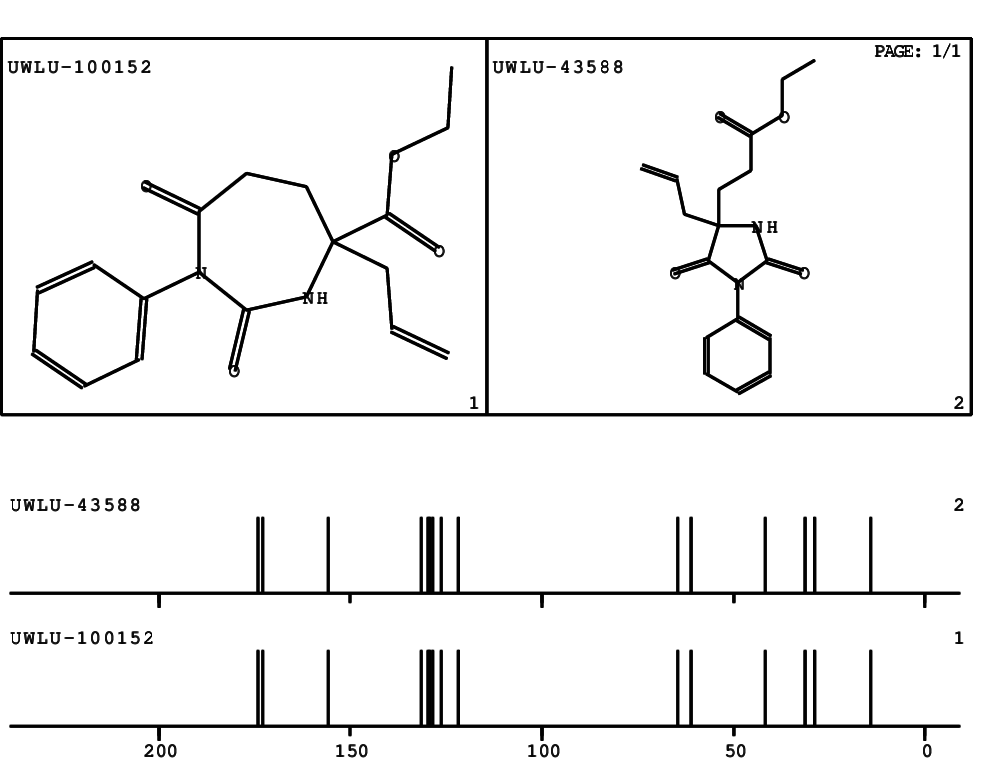
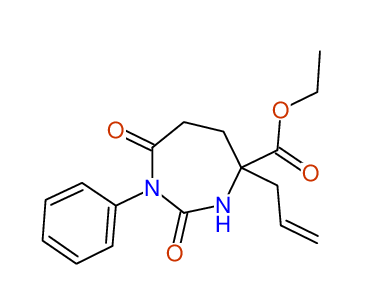
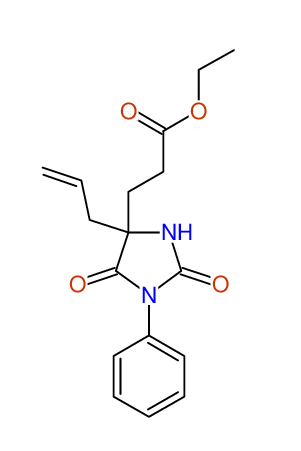
CSEARCH: UWLU-100152
ETHYL-4-ALLYL-2,7-DIOXO-1-PHENYL-1,3-DIAZEPANE-4-CARBOXYLATE
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043588
Compound 13B
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
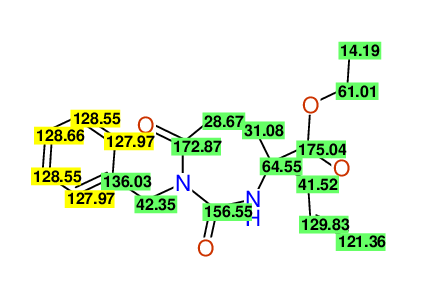
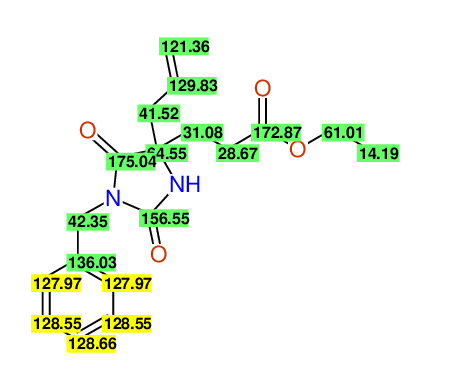
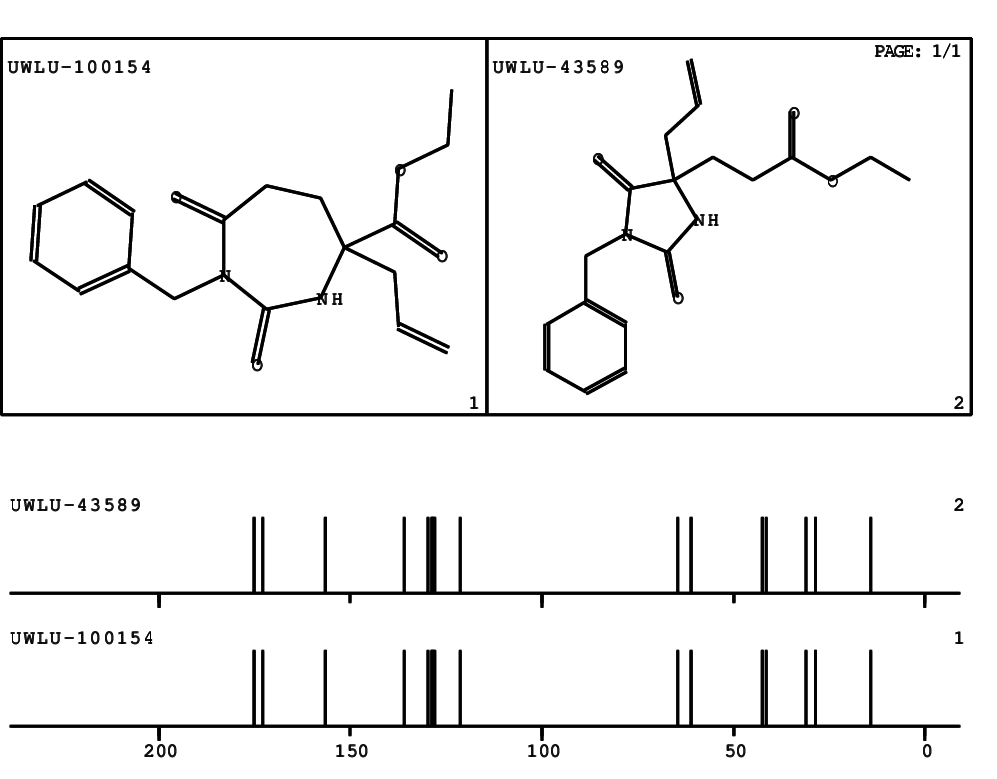
CSEARCH: UWLU-100154
ETHYL-4-ALLYL-2,7-DIOXO-1-BENZYL-1,3-DIAZEPANE-4-CARBOXYLATE
Chem.Commun., 2005, 4477 - 4478 |
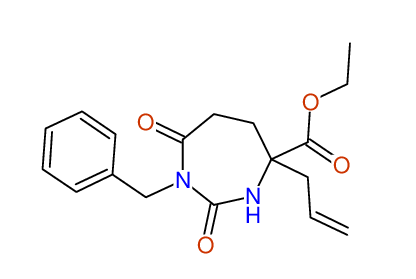
CSEARCH: UWLU-043589
Compound 13C
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
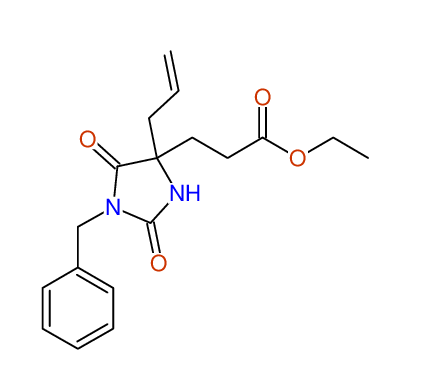 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
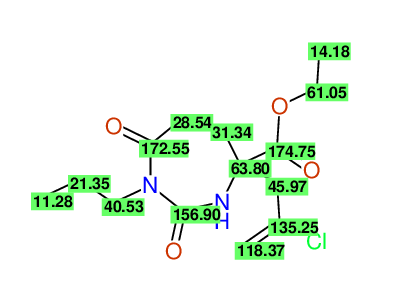
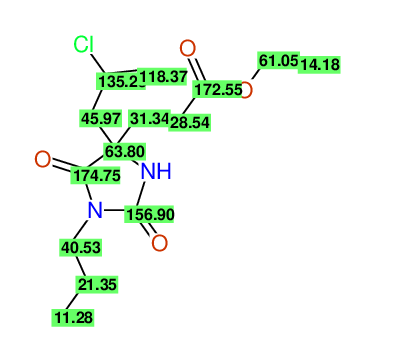
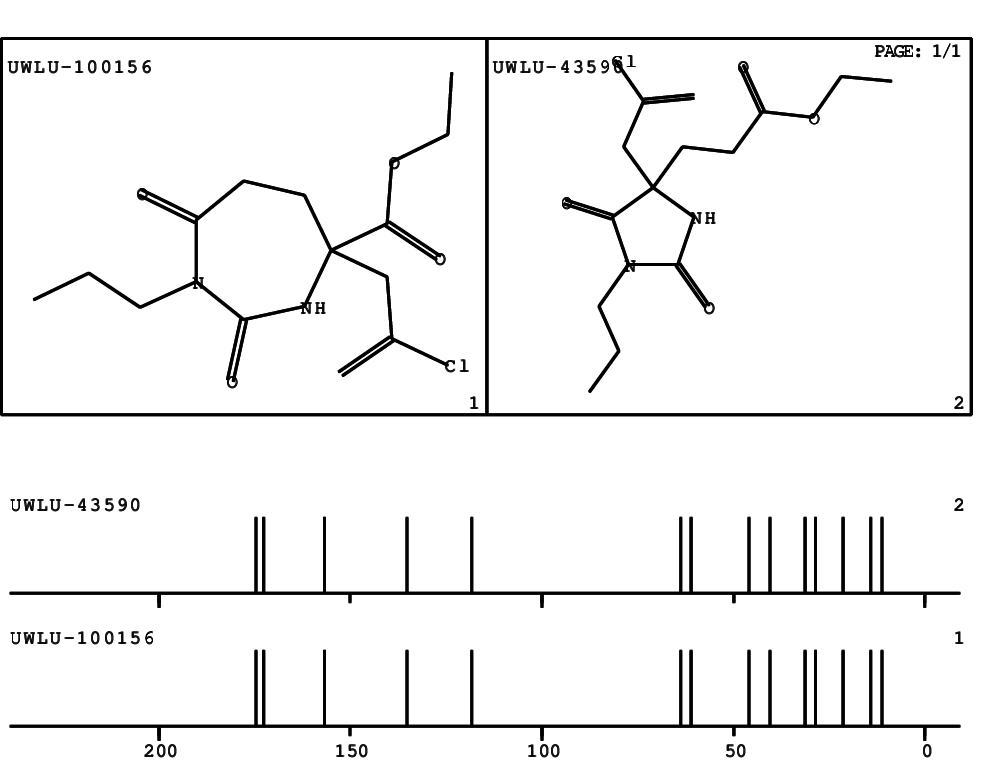
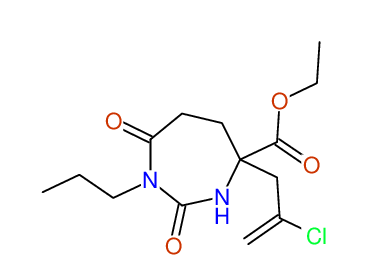
CSEARCH: UWLU-100156
ETHYL-4-[2-CHLOROPROP-2-ENYL]-2,7-DIOXO-1-PROPYL-1,3-DIAZEPANE-4-CARBOXYLATE
Chem.Commun., 2005, 4477 - 4478 |
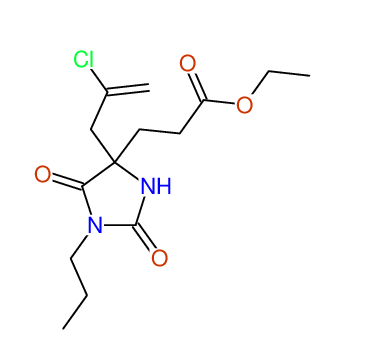
CSEARCH: UWLU-043590
Compound 13D
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
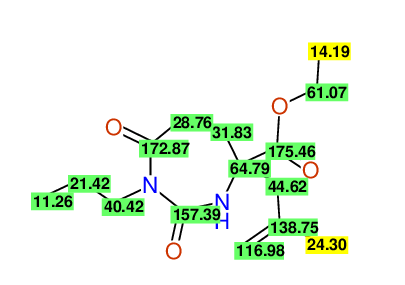
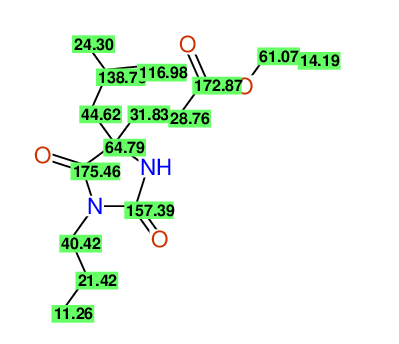
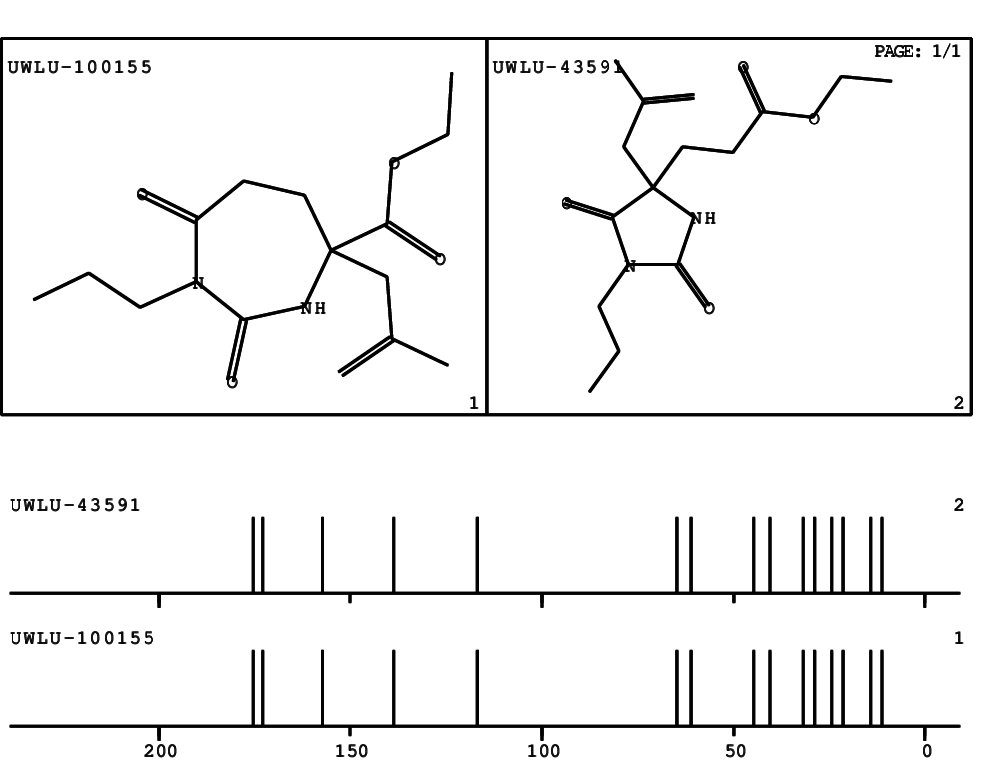
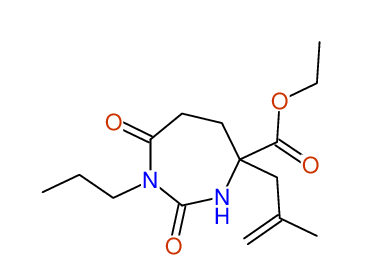
CSEARCH: UWLU-100155
ETHYL-4-[2-METHYLPROP-2-ENYL]-2,7-DIOXO-1-PROPYL-1,3-DIAZEPANE-4-CARBOXYLATE
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043591
Compound 13E
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
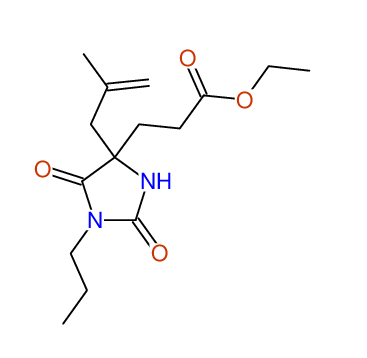 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
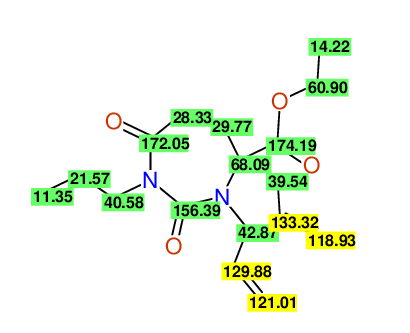
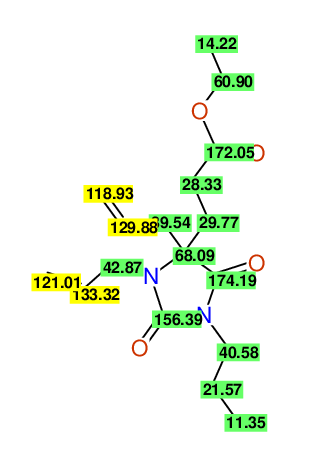
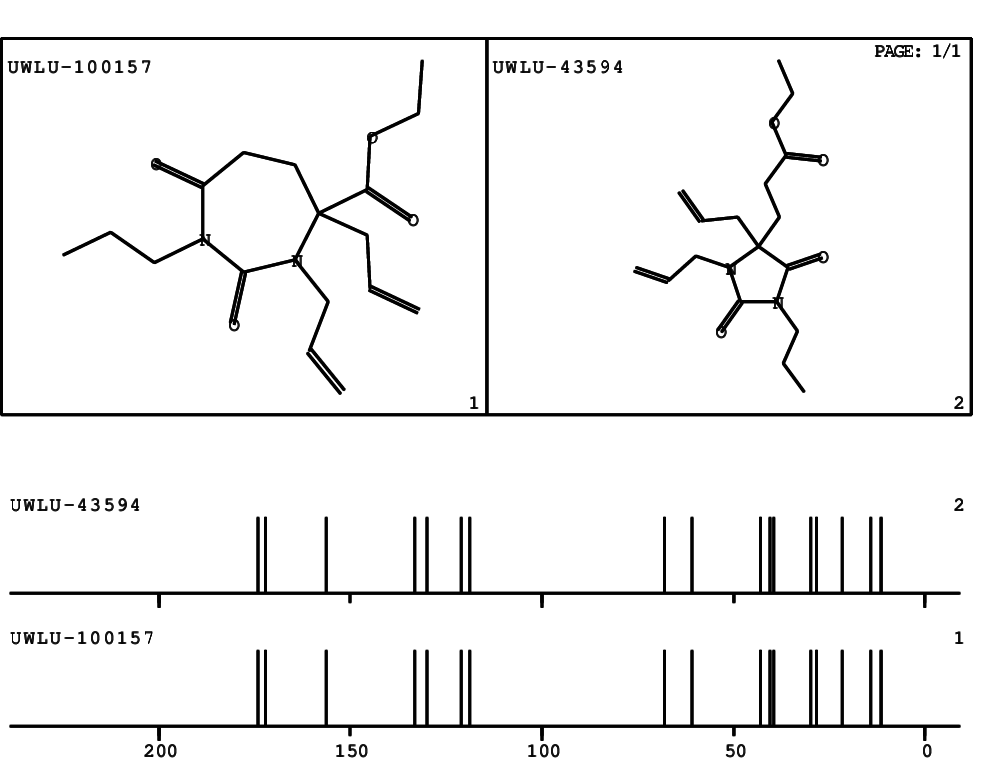
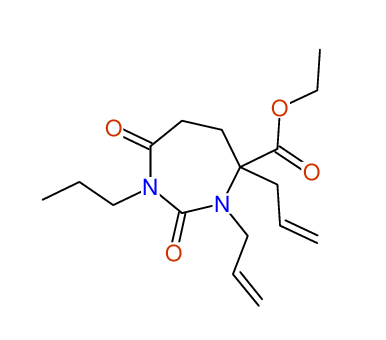
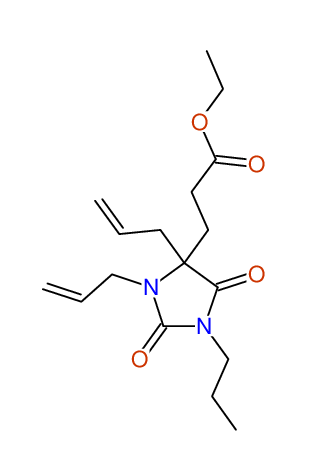
CSEARCH: UWLU-100157
Ethyl 3,4-diallyl-2,7-dioxo-1-propyl-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043594
Compound 14A
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
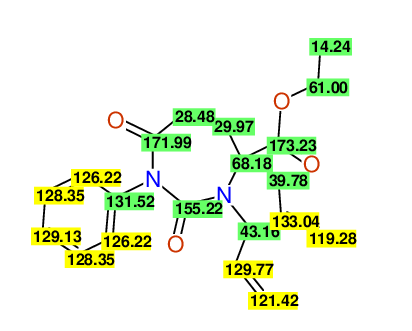
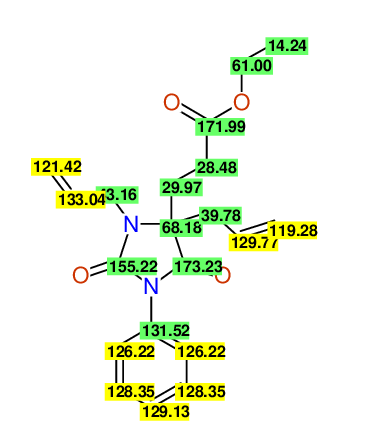
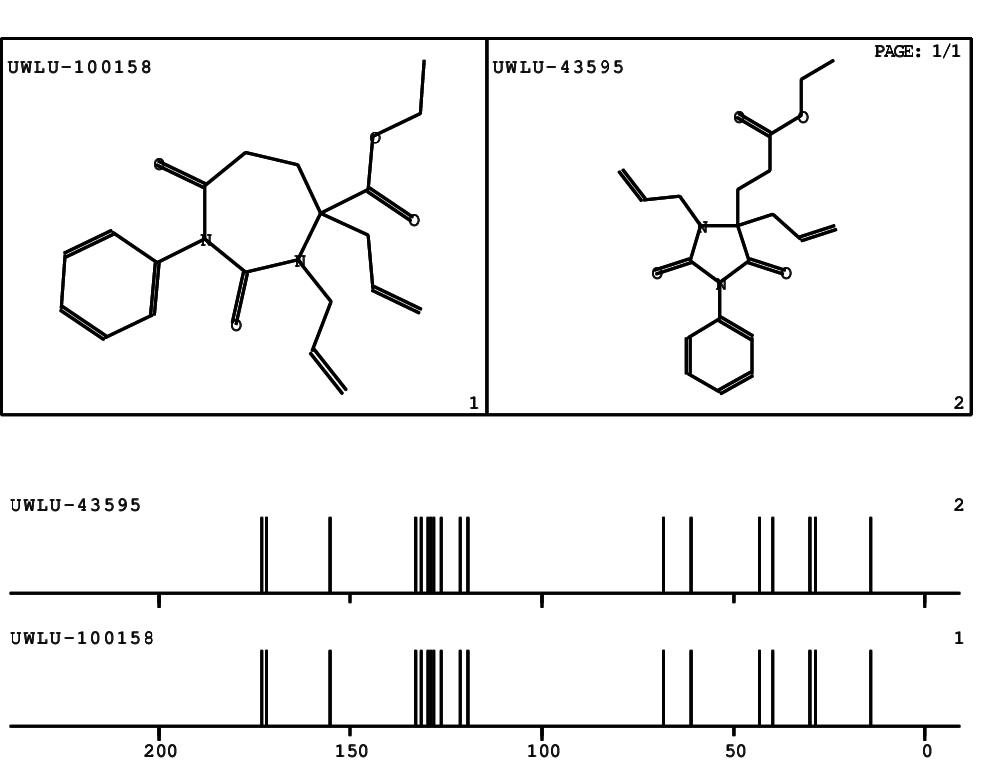
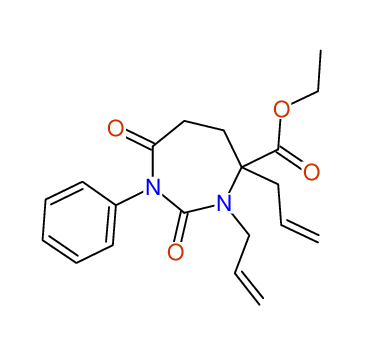
CSEARCH: UWLU-100158
Ethyl 3,4-diallyl-2,7-dioxo-1-phenyl-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043595
Compound 14B
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
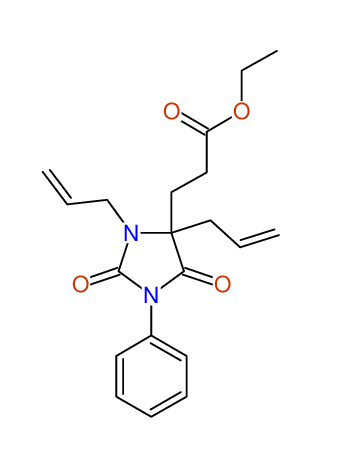 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
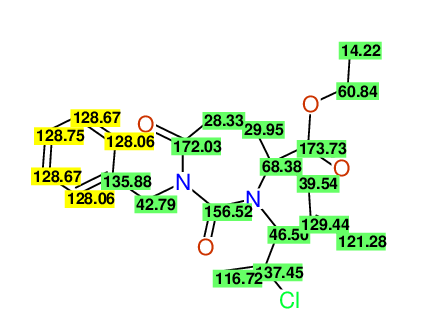
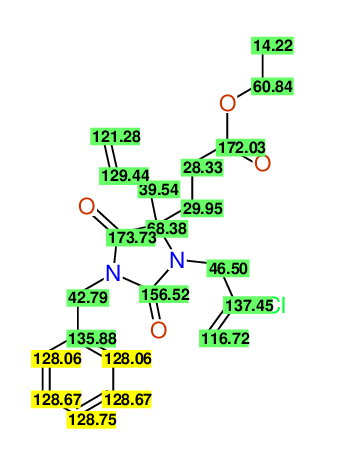
CSEARCH: UWLU-100160
Ethyl 4-allyl-1-benzyl-3-(2-chloroprop-2-enyl)-2,7-dioxo-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043596
Compound 14C
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
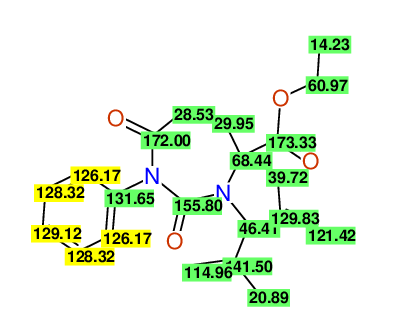
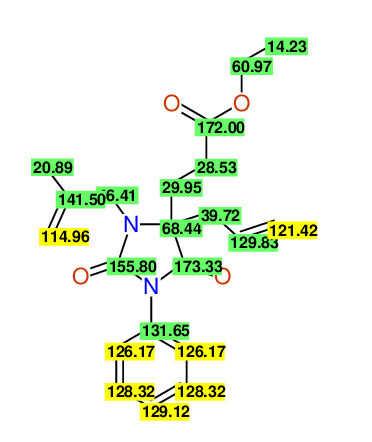
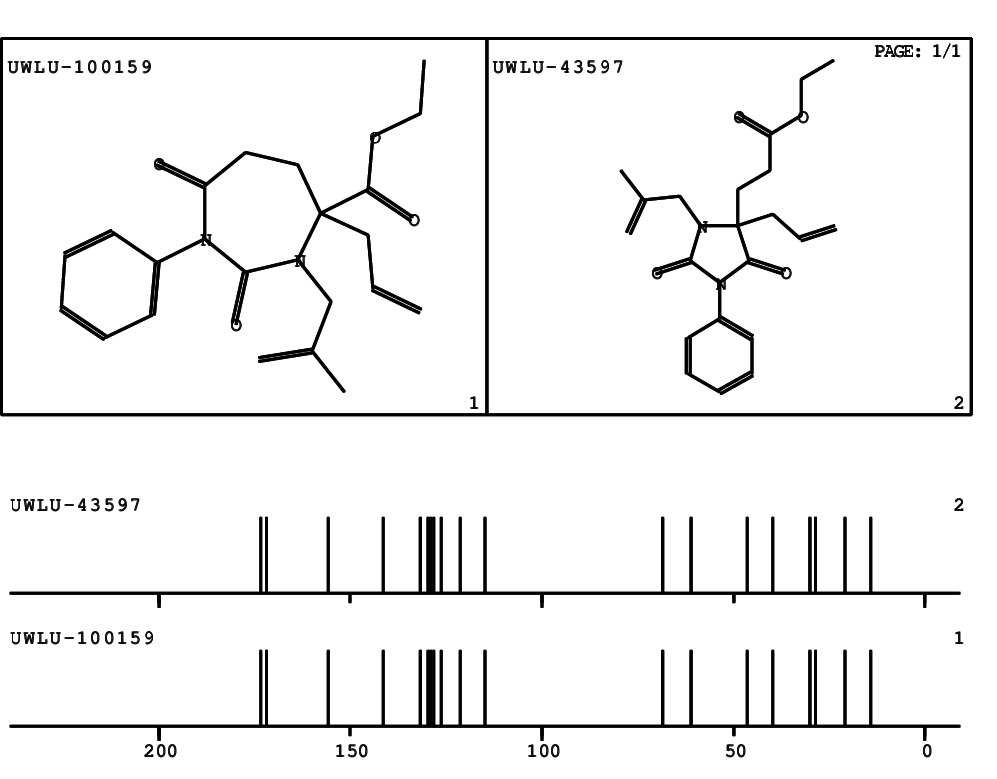
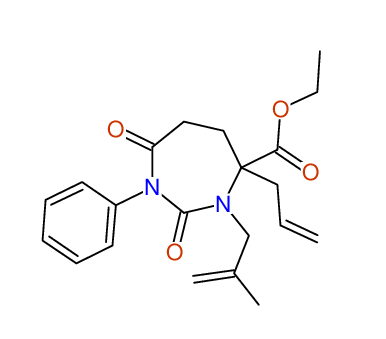
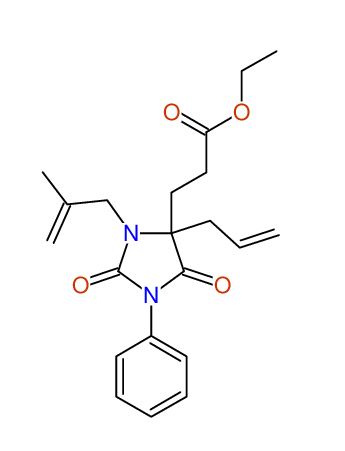
CSEARCH: UWLU-100159
Ethyl 4-allyl-3-(2-methylprop-2-enyl)-1-phenyl-2,7-dioxo-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043597
Compound 14D
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
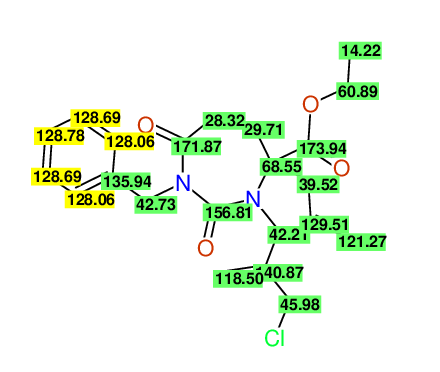
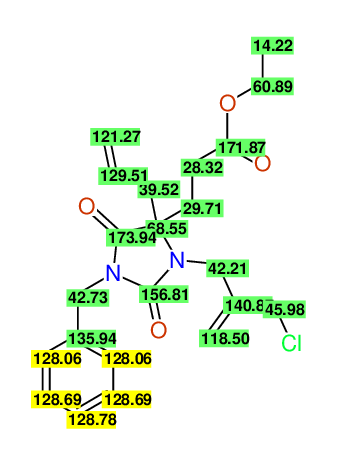
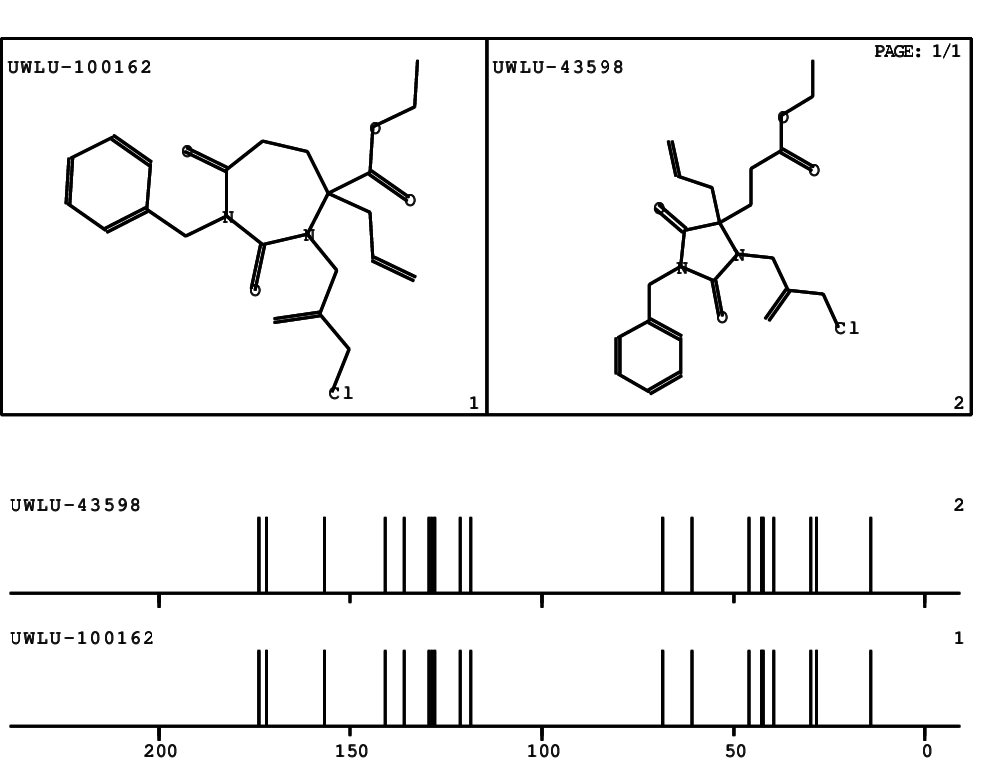
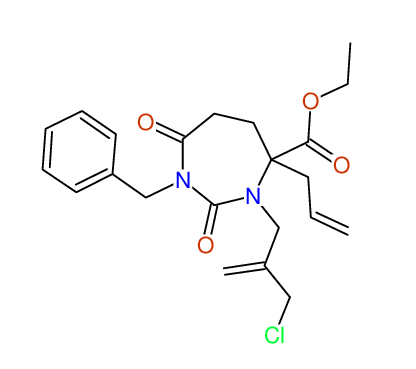
CSEARCH: UWLU-100162
Ethyl 4-allyl-1-benzyl-3-(2-chloroprop-2-enyl)-2,7-dioxo-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043598
Compound 14E
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
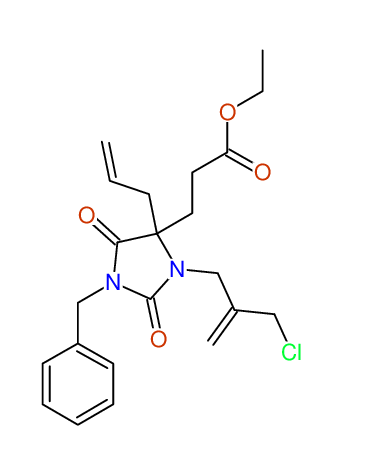
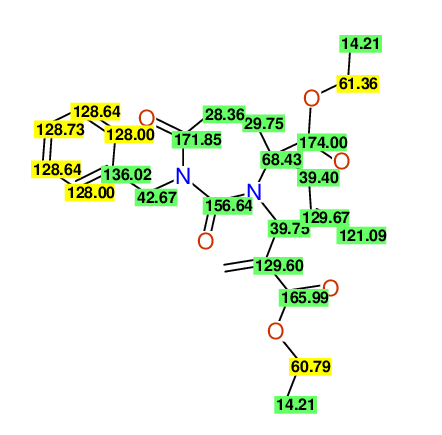
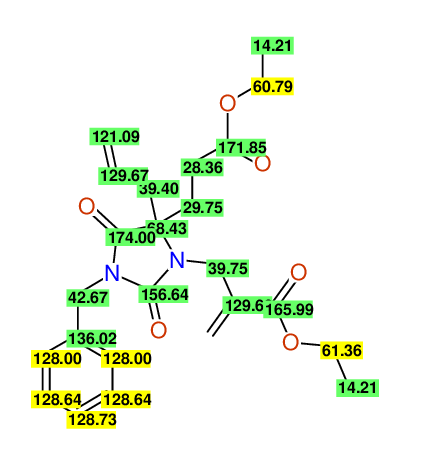
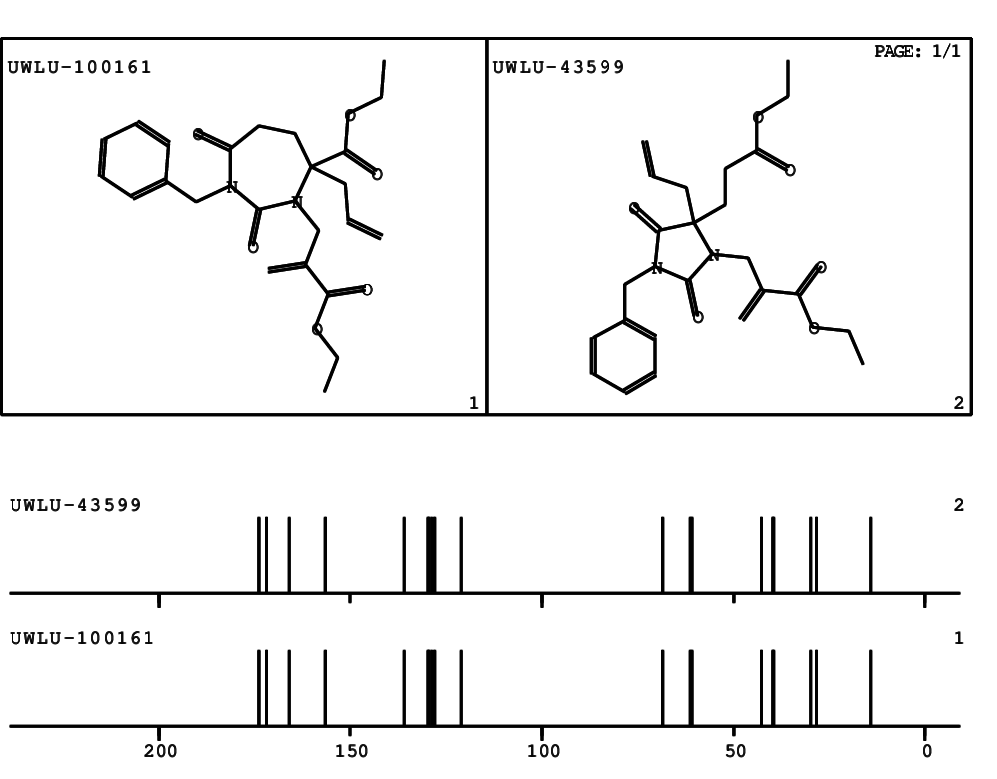
CSEARCH: UWLU-100161
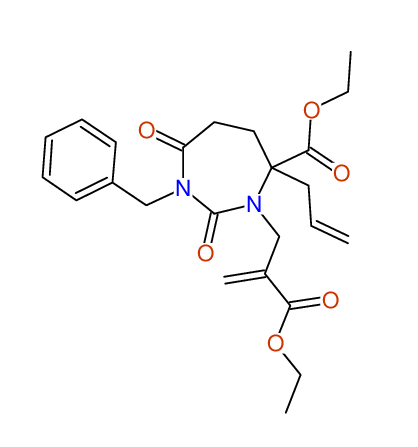
Ethyl-4-allyl-1-benzyl-3-[2-(ethoxycarbonyl)prop-2-enyl]-2,7-dioxo-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043599
Compound 14F
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
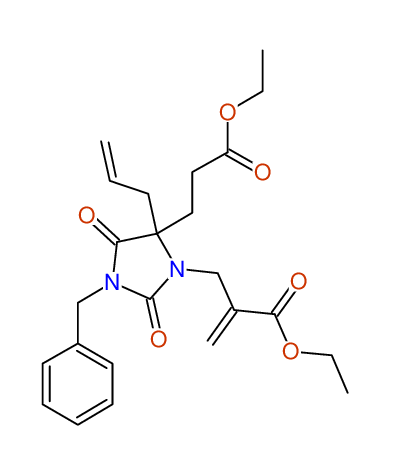 |
- Identical 1H-NMR data
- Chemical shift values are always sorted - both peaklists show a sequence of 2.60 / 2.52 / 4.02-4.16 ppm
- Identical 13C-NMR data
- In both 13C-NMR data sets 1 signal is missing - 2 olefinic CH2
in structure; only 1 signal is given
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
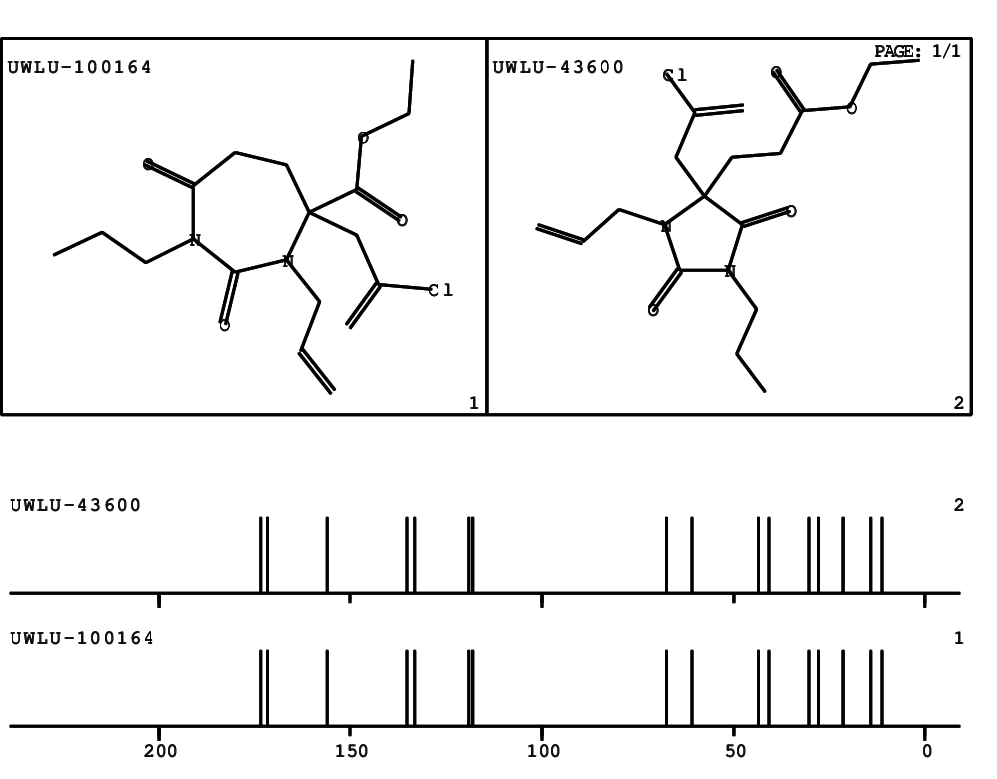
CSEARCH: UWLU-100164
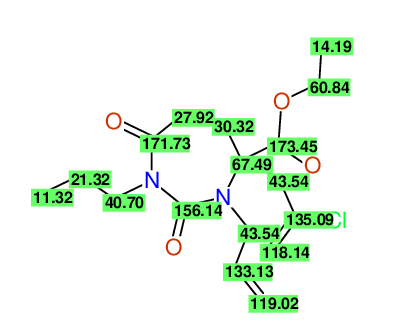
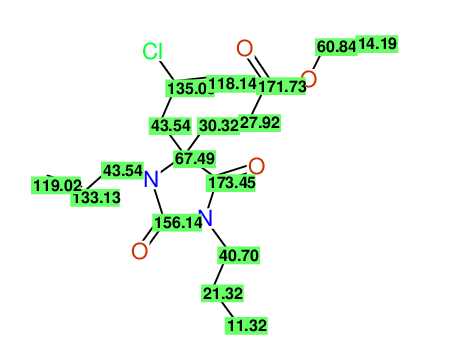
Ethyl 3-allyl-4-(2-chloroprop-2-enyl)-1-propyl-2,7-dioxo-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043600
Compound 14G
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
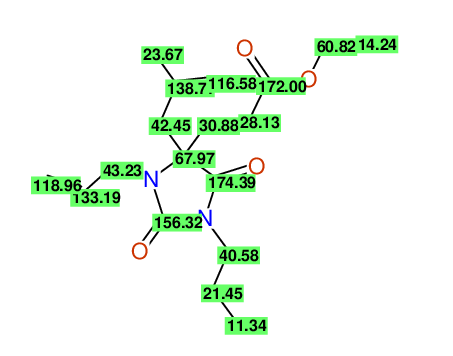
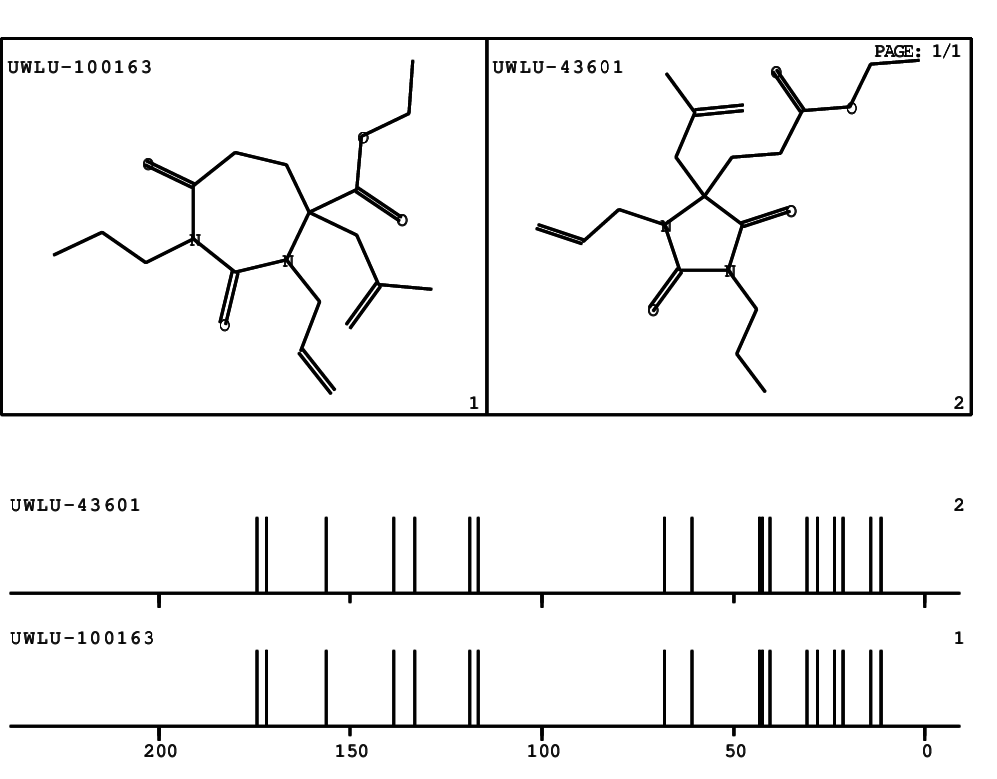
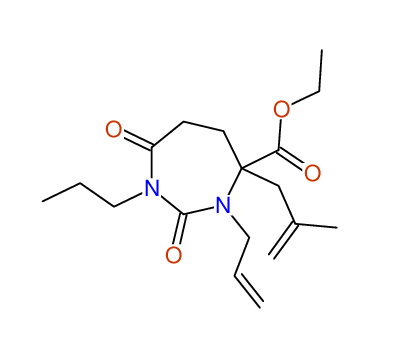
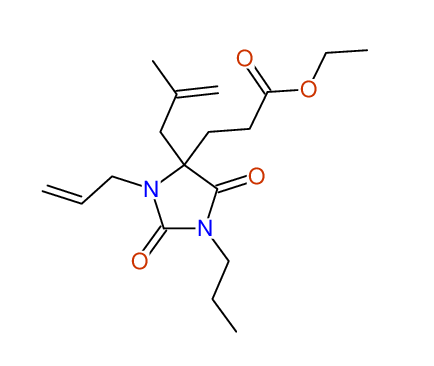
CSEARCH: UWLU-100163
Ethyl 3-allyl-4-(2-chloroprop-2-enyl)-1-propyl-2,7-dioxo-1,3-diazepane-4-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043601
Compound 14H
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
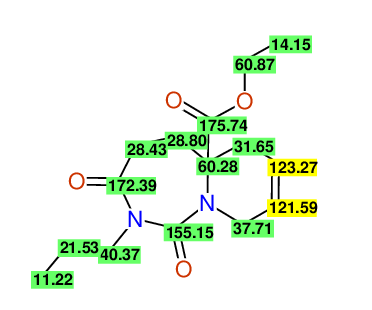
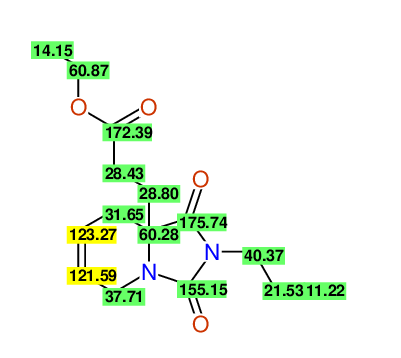
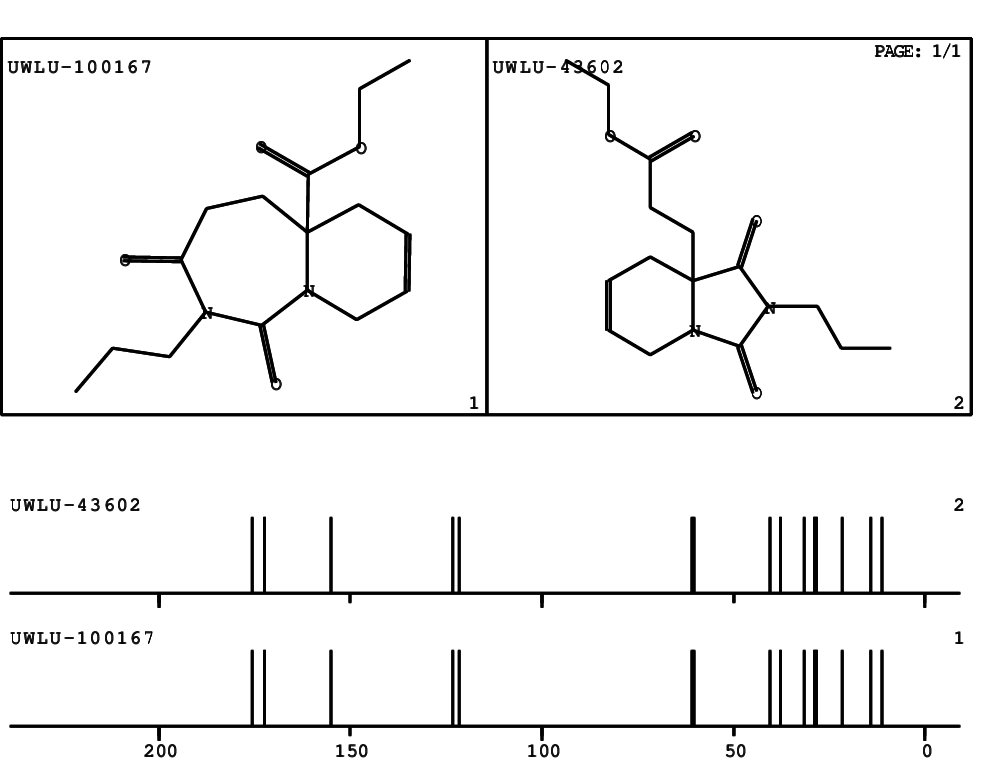
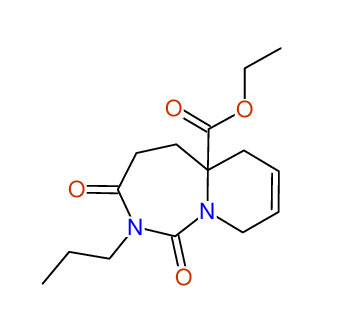
CSEARCH: UWLU-100167
Ethyl-1,3-dioxo-2-propyl-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043602
Compound 15A
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
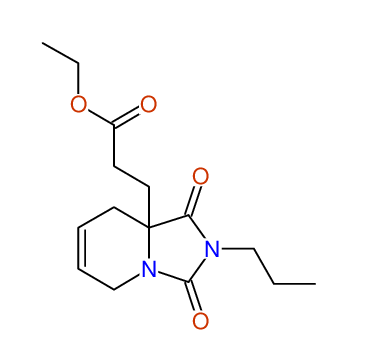 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
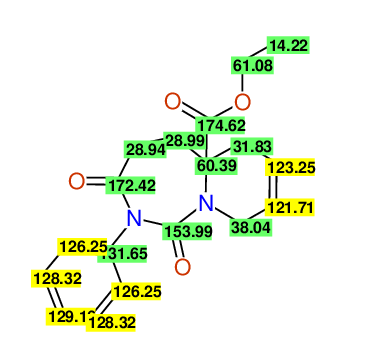
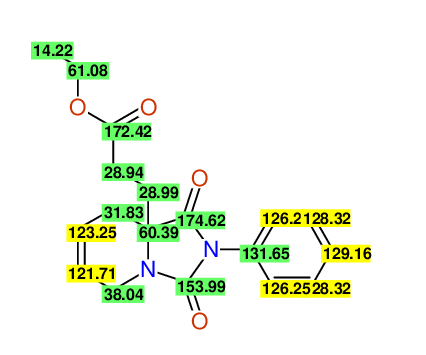
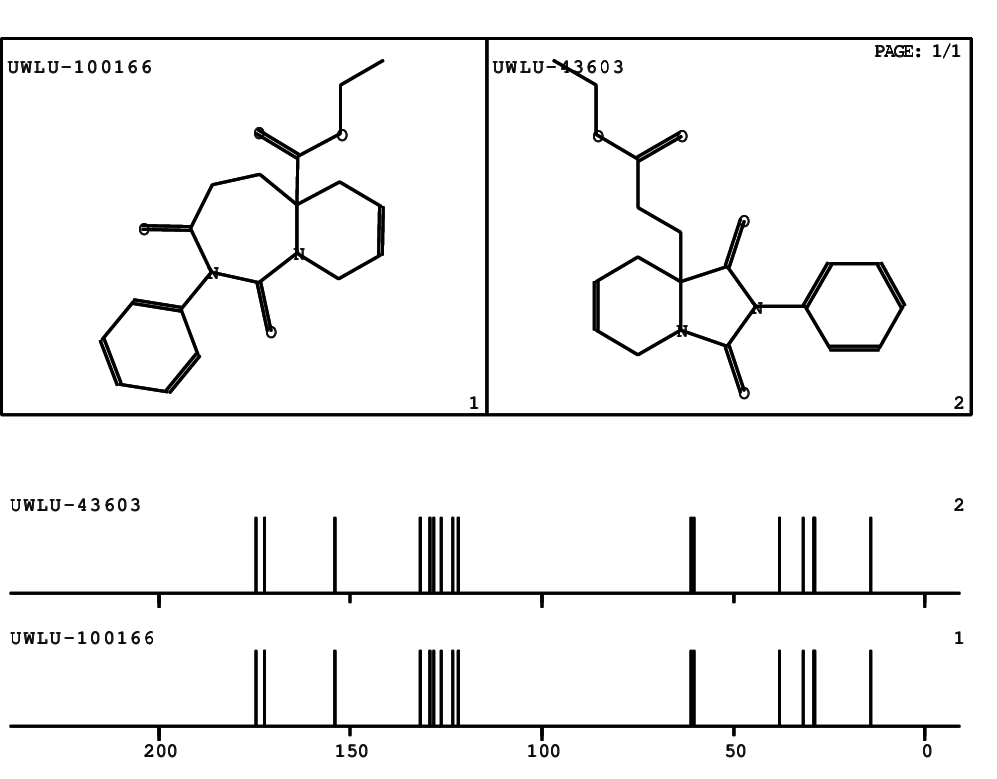
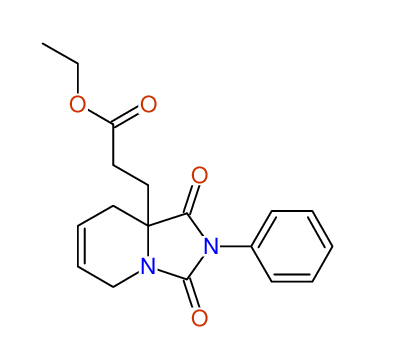
CSEARCH: UWLU-100166
Ethyl-1,3-dioxo-2-phenyl-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043603
Compound 15B
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
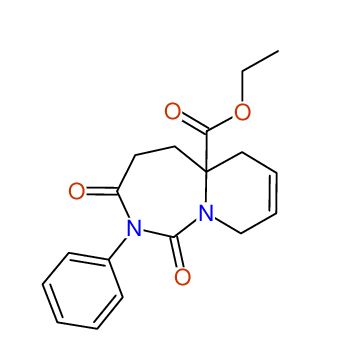
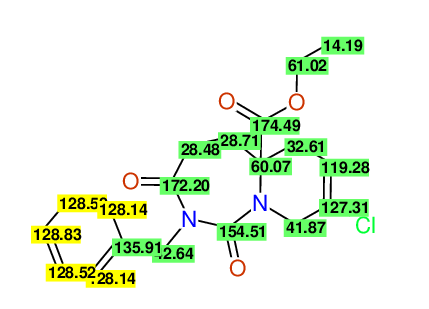
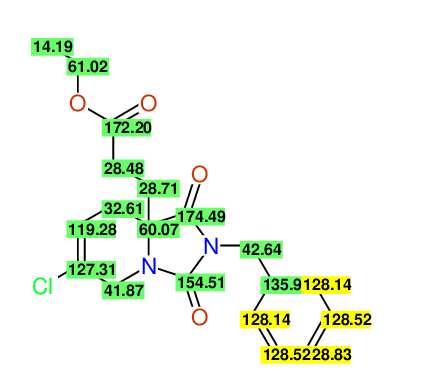
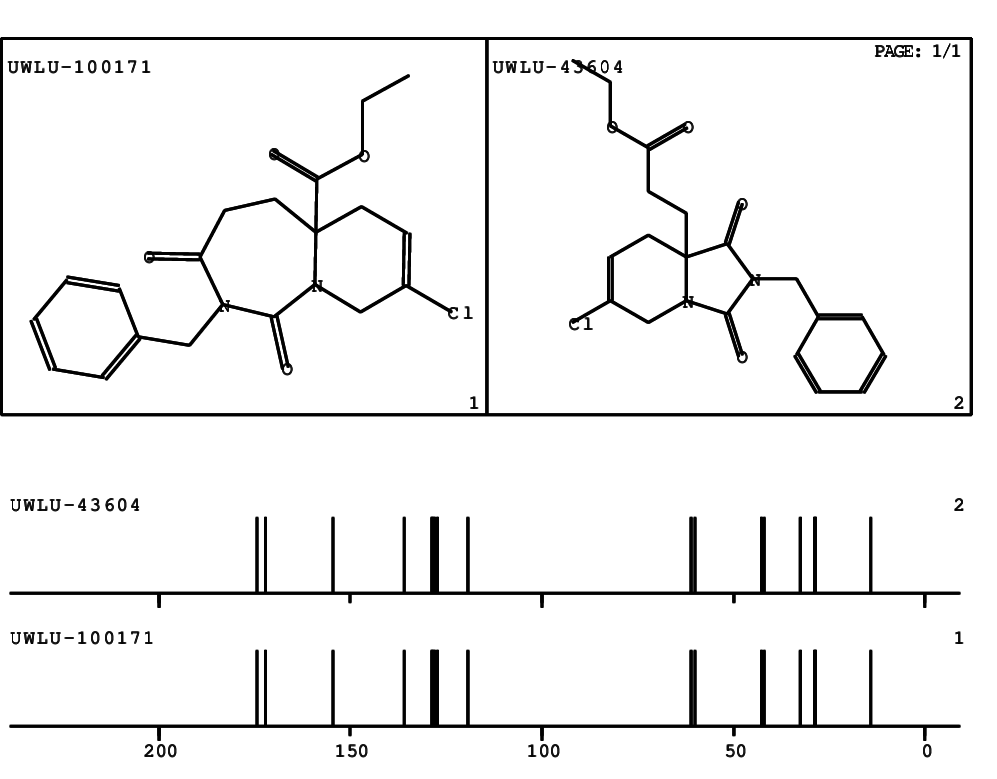
CSEARCH: UWLU-100171
Ethyl 2-benzyl-8-chloro-1,3-dioxo-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-
carboxylate
Chem.Commun., 2005, 4477 - 4478 |
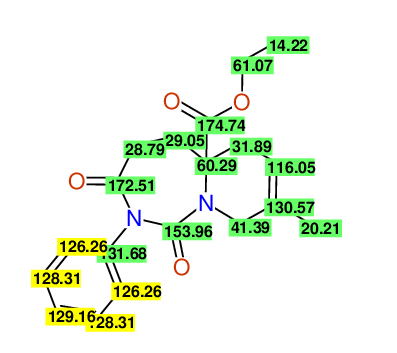
CSEARCH: UWLU-043604
Compound 15C
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
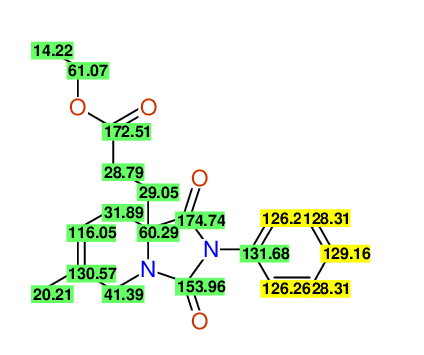
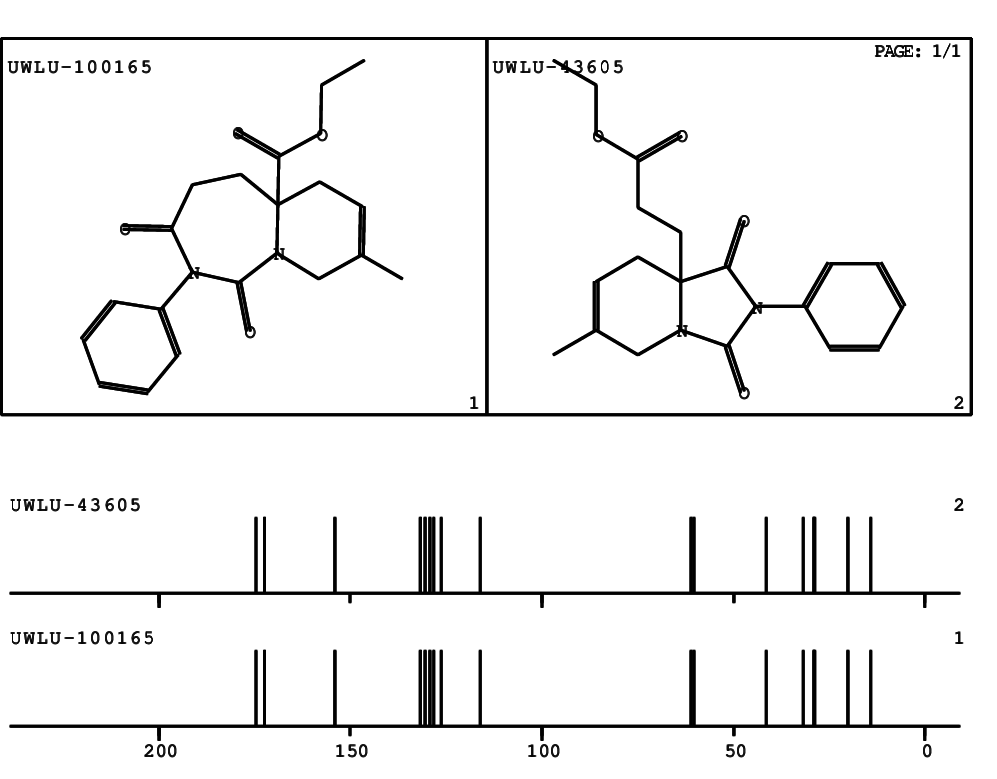
CSEARCH: UWLU-100165
Ethyl 8-methyl-1,3-dioxo-2-phenyl-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043605
Compound 15D
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
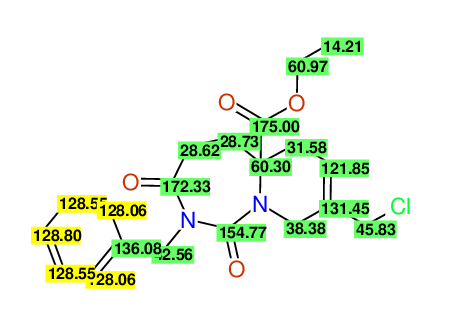
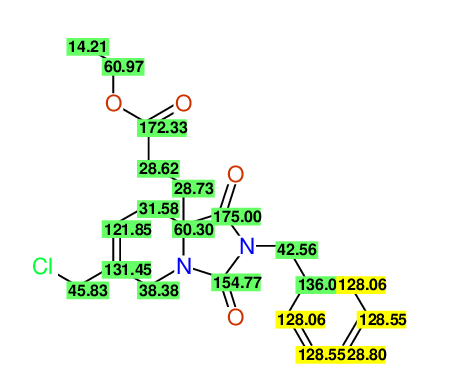
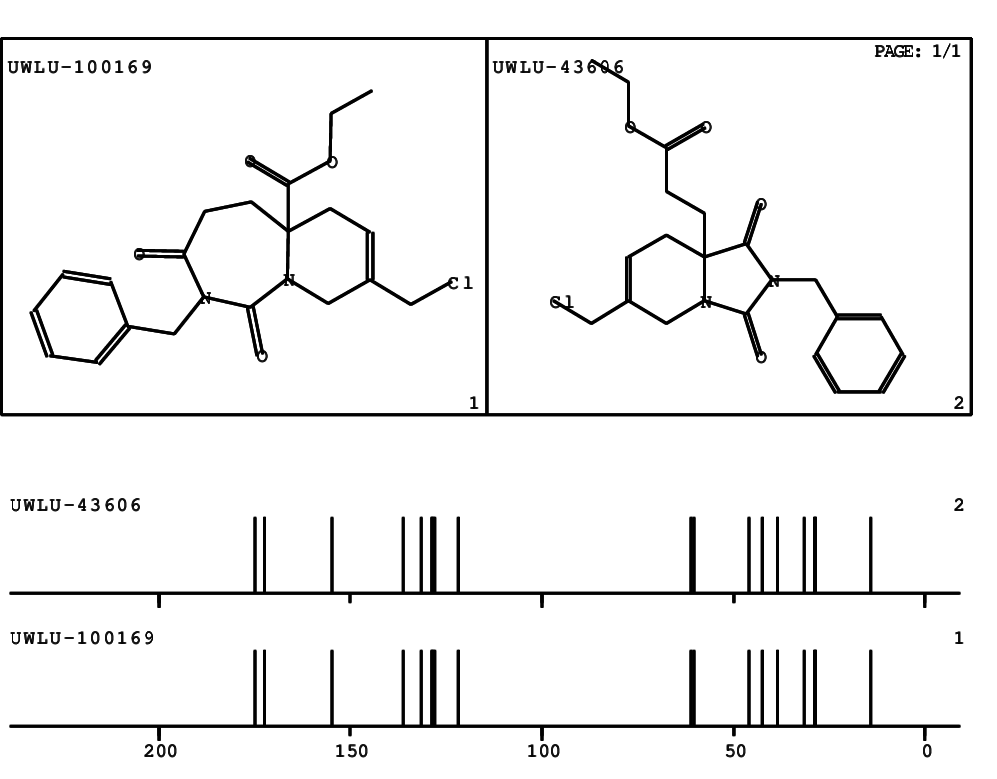
CSEARCH: UWLU-100169
Ethyl 2-benzyl-8-(chloromethyl)-1,3-dioxo-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-43606
Compound 15E
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
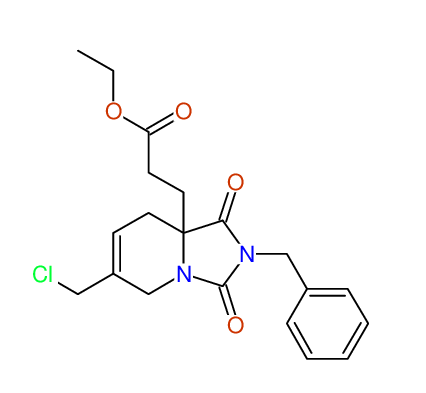 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
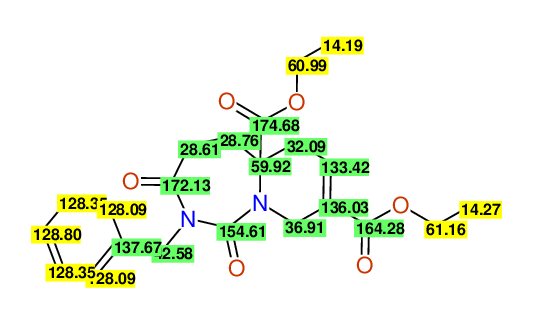
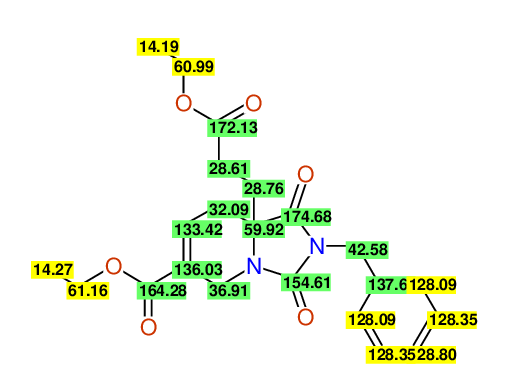
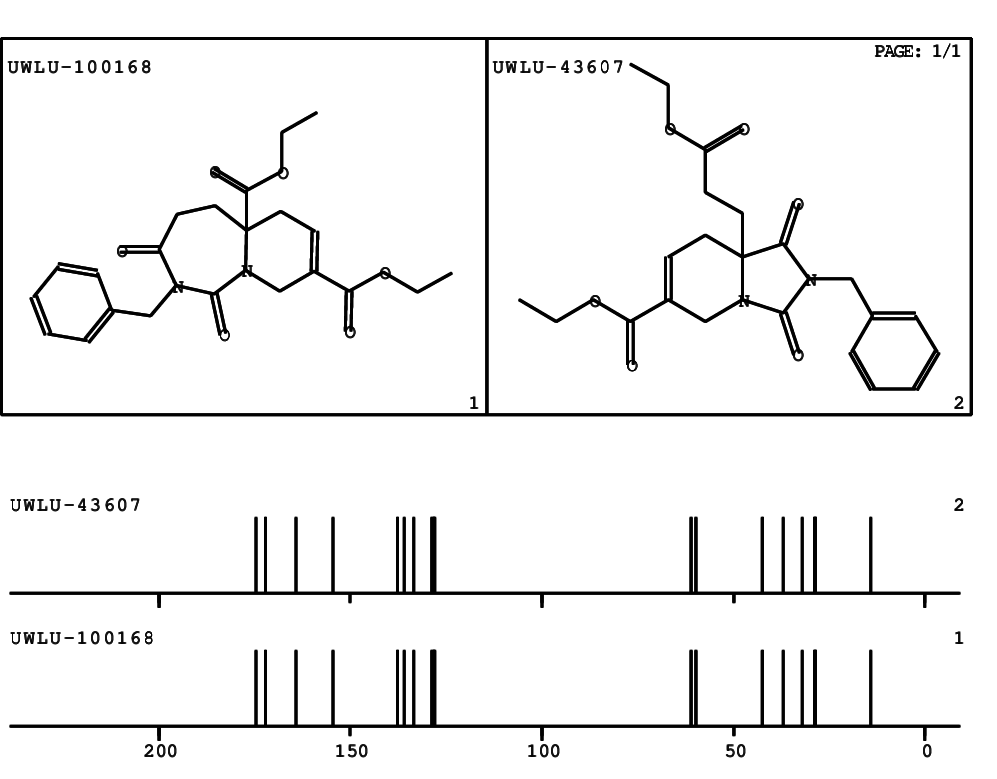
CSEARCH: UWLU-100168
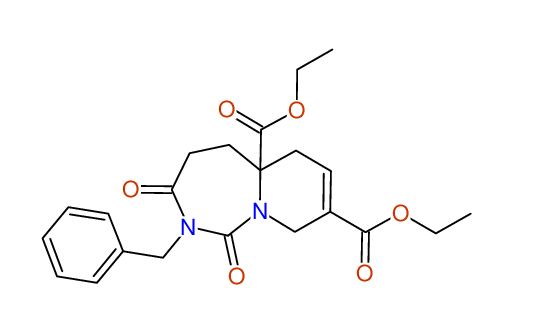
Diethyl 2-benzyl-1,3-dioxo-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a,8(1H)-dicarboxylate
Chem.Commun., 2005, 4477 - 4478
"Instead of the second generation GrubbsTM catalyst,
the second generation Hoveyda-GrubbsTM
catalyst was used for this reaction"
"Chromatography:
first Hex/EtOAc (3/1) untill Rf = 0.26, then strip with EtOAc + 5% CH2Cl2"
|
CSEARCH: UWLU-043607
Compound 15F
Eur.J.Org.Chem., 2006, 2649-2660
"Instead of the
second generation GrubbsTM catalyst, the second generation
Hoveyda-GrubbsTM catalyst is used for this reaction"
"Chromatography: first
Hex/EtOAc (3:1) until Rf = 0.26, then strip with EtOAc + 5 %
CH2Cl2"
|
Schematic spectra |
 |
 |
 |
 |
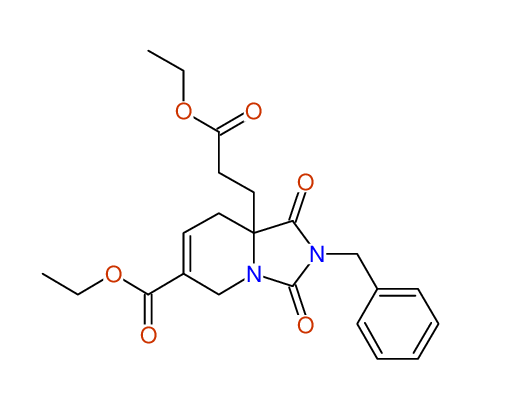 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|
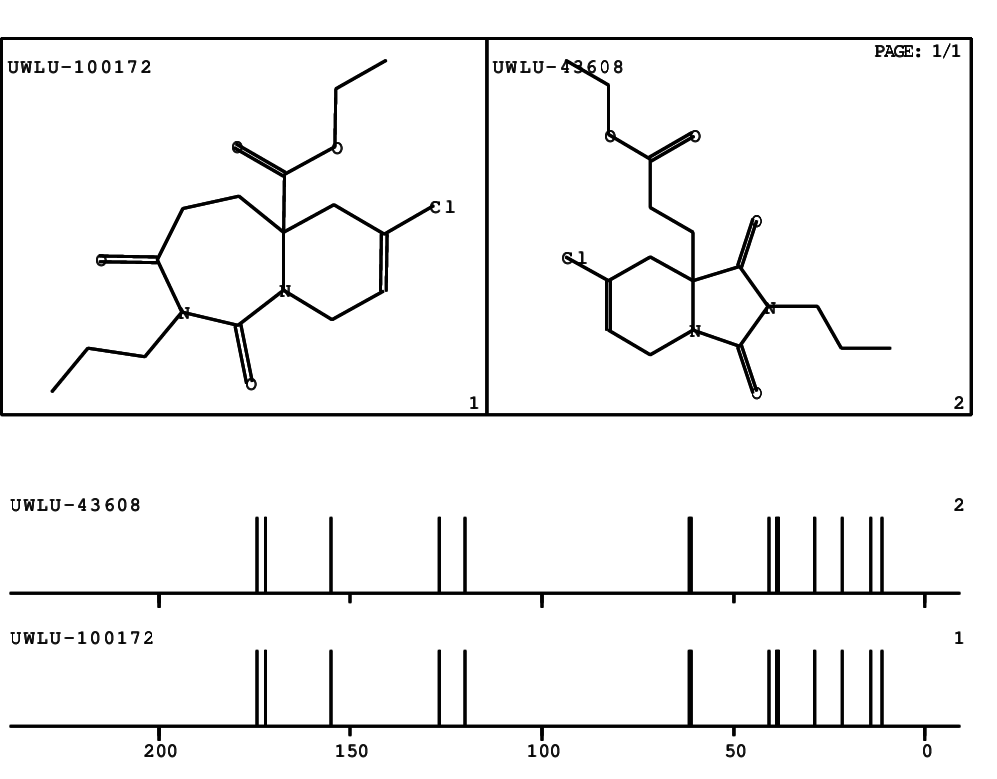
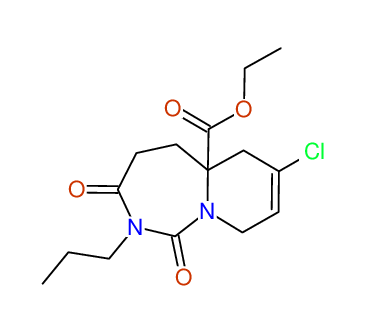
CSEARCH: UWLU-100172
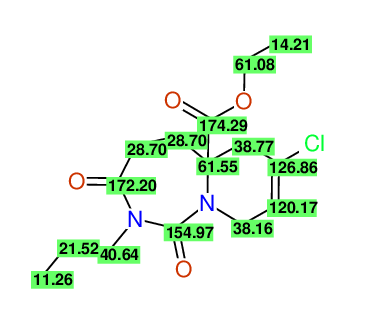
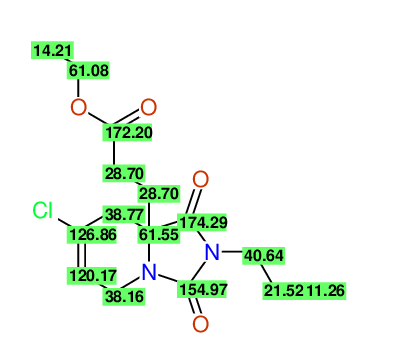
Ethyl 7-chloro-1,3-dioxo-2-propyl-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-
carboxylate
Chem.Commun., 2005, 4477 - 4478 |
CSEARCH: UWLU-043608
Compound 15G
Eur.J.Org.Chem., 2006, 2649-2660 |
Schematic spectra |
 |
 |
 |
 |
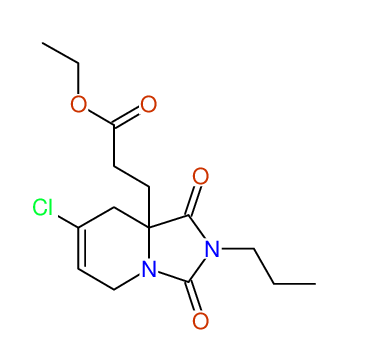 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
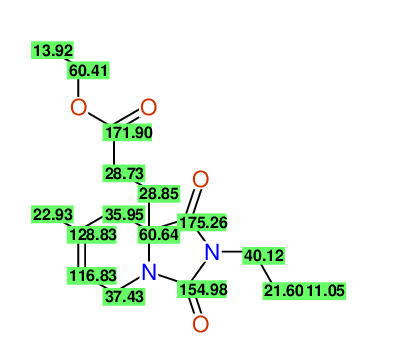
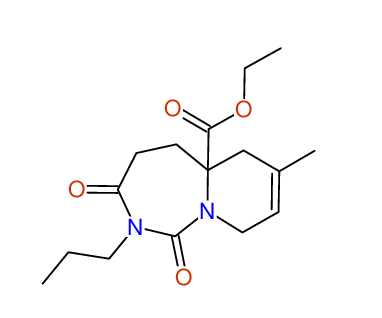
CSEARCH: UWLU-100170
Ethyl 7-methyl-1,3-dioxo-2-propyl-2,3,4,5,6,9-hexahydropyrido[1,2-c][1,3]diazepine-5a(1H)-
carboxylate
Chem.Commun., 2005, 4477 - 4478 |
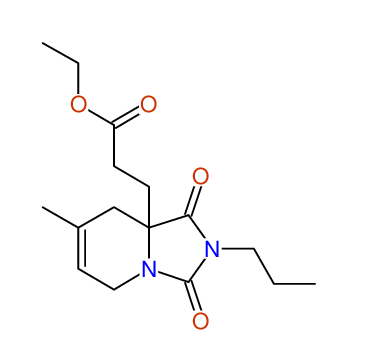
CSEARCH: UWLU-043609
Compound 15H
Eur.J.Org.Chem., 2006, 2649-2660 |
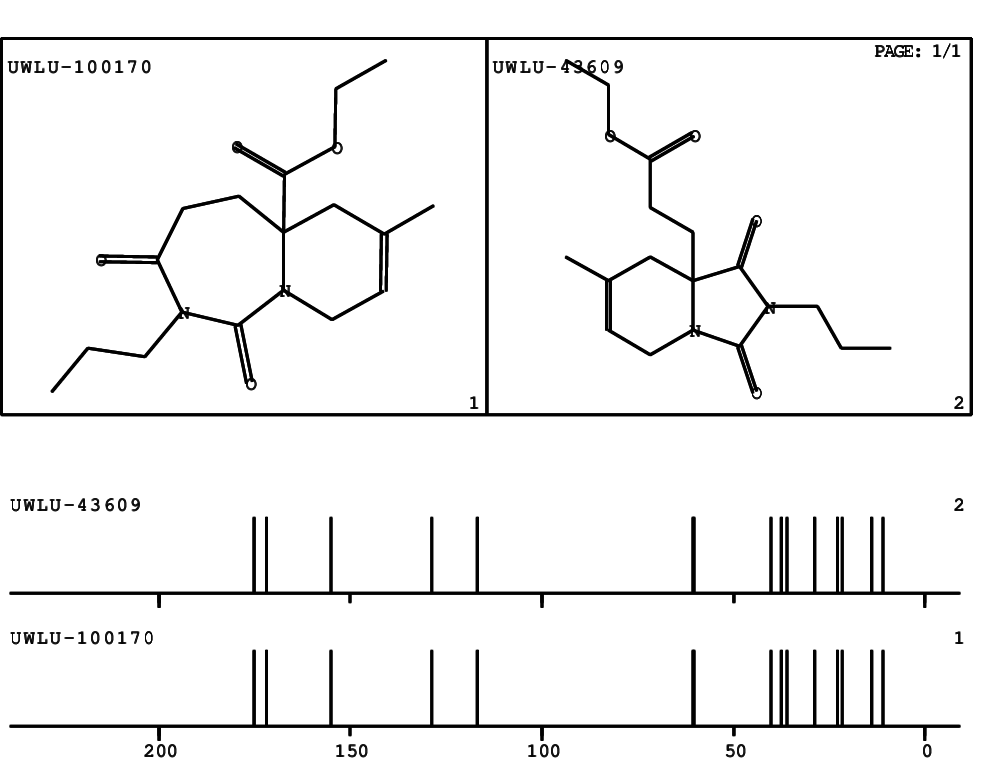
Schematic spectra |
|
 |
 |
 |
 |
 |
- Identical 1H-NMR data
- Identical 13C-NMR data
- Identical IR-data
- Structure revision
- Original literature not cited
|
|

















































































































































